Effect of Lower Tidal Volume Ventilation Facilitated by Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure: The REST Randomized Clinical Trial
- PMID: 34463700
- PMCID: PMC8408762
- DOI: 10.1001/jama.2021.13374
Effect of Lower Tidal Volume Ventilation Facilitated by Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure: The REST Randomized Clinical Trial
Erratum in
-
Error in Figures.JAMA. 2022 Jan 4;327(1):86. doi: 10.1001/jama.2021.21948. JAMA. 2022. PMID: 34910082 Free PMC article. No abstract available.
Abstract
Importance: In patients who require mechanical ventilation for acute hypoxemic respiratory failure, further reduction in tidal volumes, compared with conventional low tidal volume ventilation, may improve outcomes.
Objective: To determine whether lower tidal volume mechanical ventilation using extracorporeal carbon dioxide removal improves outcomes in patients with acute hypoxemic respiratory failure.
Design, setting, and participants: This multicenter, randomized, allocation-concealed, open-label, pragmatic clinical trial enrolled 412 adult patients receiving mechanical ventilation for acute hypoxemic respiratory failure, of a planned sample size of 1120, between May 2016 and December 2019 from 51 intensive care units in the UK. Follow-up ended on March 11, 2020.
Interventions: Participants were randomized to receive lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal for at least 48 hours (n = 202) or standard care with conventional low tidal volume ventilation (n = 210).
Main outcomes and measures: The primary outcome was all-cause mortality 90 days after randomization. Prespecified secondary outcomes included ventilator-free days at day 28 and adverse event rates.
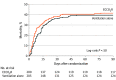
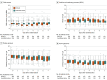
Results: Among 412 patients who were randomized (mean age, 59 years; 143 [35%] women), 405 (98%) completed the trial. The trial was stopped early because of futility and feasibility following recommendations from the data monitoring and ethics committee. The 90-day mortality rate was 41.5% in the lower tidal volume ventilation with extracorporeal carbon dioxide removal group vs 39.5% in the standard care group (risk ratio, 1.05 [95% CI, 0.83-1.33]; difference, 2.0% [95% CI, -7.6% to 11.5%]; P = .68). There were significantly fewer mean ventilator-free days in the extracorporeal carbon dioxide removal group compared with the standard care group (7.1 [95% CI, 5.9-8.3] vs 9.2 [95% CI, 7.9-10.4] days; mean difference, -2.1 [95% CI, -3.8 to -0.3]; P = .02). Serious adverse events were reported for 62 patients (31%) in the extracorporeal carbon dioxide removal group and 18 (9%) in the standard care group, including intracranial hemorrhage in 9 patients (4.5%) vs 0 (0%) and bleeding at other sites in 6 (3.0%) vs 1 (0.5%) in the extracorporeal carbon dioxide removal group vs the control group. Overall, 21 patients experienced 22 serious adverse events related to the study device.
Conclusions and relevance: Among patients with acute hypoxemic respiratory failure, the use of extracorporeal carbon dioxide removal to facilitate lower tidal volume mechanical ventilation, compared with conventional low tidal volume mechanical ventilation, did not significantly reduce 90-day mortality. However, due to early termination, the study may have been underpowered to detect a clinically important difference.
Trial registration: ClinicalTrials.gov Identifier: NCT02654327.
Conflict of interest statement
Figures
Comment in
-
Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation Effect on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure.JAMA. 2022 Jan 4;327(1):82. doi: 10.1001/jama.2021.21002. JAMA. 2022. PMID: 34982124 No abstract available.
-
Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation Effect on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure.JAMA. 2022 Jan 4;327(1):82-83. doi: 10.1001/jama.2021.20999. JAMA. 2022. PMID: 34982125 No abstract available.
-
Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation Effect on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure.JAMA. 2022 Jan 4;327(1):83-84. doi: 10.1001/jama.2021.20996. JAMA. 2022. PMID: 34982126 No abstract available.
-
Conditional Power: How Likely Is Trial Success?JAMA. 2023 Feb 14;329(6):508-509. doi: 10.1001/jama.2022.25080. JAMA. 2023. PMID: 36689237
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. - PubMed
-
- Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. doi: 10.1056/NEJM200005043421801 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

