Gating pore currents occur in CaV1.1 domain III mutants associated with HypoPP
- PMID: 34463712
- PMCID: PMC8563280
- DOI: 10.1085/jgp.202112946
Gating pore currents occur in CaV1.1 domain III mutants associated with HypoPP
Abstract
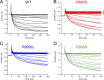
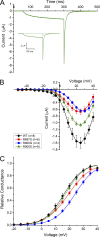
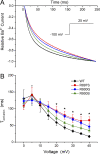
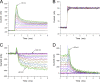
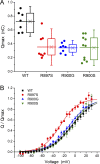
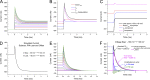
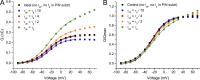
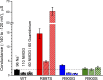
Mutations in the voltage sensor domain (VSD) of CaV1.1, the α1S subunit of the L-type calcium channel in skeletal muscle, are an established cause of hypokalemic periodic paralysis (HypoPP). Of the 10 reported mutations, 9 are missense substitutions of outer arginine residues (R1 or R2) in the S4 transmembrane segments of the homologous domain II, III (DIII), or IV. The prevailing view is that R/X mutations create an anomalous ion conduction pathway in the VSD, and this so-called gating pore current is the basis for paradoxical depolarization of the resting potential and weakness in low potassium for HypoPP fibers. Gating pore currents have been observed for four of the five CaV1.1 HypoPP mutant channels studied to date, the one exception being the charge-conserving R897K in R1 of DIII. We tested whether gating pore currents are detectable for the other three HypoPP CaV1.1 mutations in DIII. For the less conserved R1 mutation, R897S, gating pore currents with exceptionally large amplitude were observed, correlating with the severe clinical phenotype of these patients. At the R2 residue, gating pore currents were detected for R900G but not R900S. These findings show that gating pore currents may occur with missense mutations at R1 or R2 in S4 of DIII and that the magnitude of this anomalous inward current is mutation specific.
© 2021 Wu et al.
Figures