Deaminative Reductive Methylation of Alkylpyridinium Salts
- PMID: 34464140
- PMCID: PMC8448964
- DOI: 10.1021/acs.orglett.1c02458
Deaminative Reductive Methylation of Alkylpyridinium Salts
Abstract
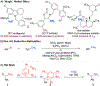
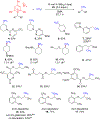
Methyl groups can imbue valuable properties in organic molecules, often leading to enhanced bioactivity. To enable efficient installation of methyl groups on simple building blocks and in late-stage functionalization, a nickel-catalyzed reductive coupling of secondary Katritzky alkylpyridinium salts with methyl iodide was developed. When coupled with formation of the pyridinium salt from an alkyl amine, this method allows amino groups to be readily transformed to methyl groups with broad functional group and heterocycle tolerance.
Figures


References
-
- Schönherr H; Cernak T, Profound Methyl Effects in Drug Discovery and a Call for New C-H Methylation Reactions. Angew. Chem., Int. Ed 2013, 52, 12256–12267. - PubMed
-
- Leung CS; Leung SSF; Tirado-Rives J; Jorgensen WL, Methyl Effects on Protein–Ligand Binding. J. Med. Chem 2012, 55, 4489–4500; - PMC - PubMed
- Barreiro EJ; Kümmerle AE; Fraga CAM, The Methylation Effect in Medicinal Chemistry. Chem. Rev 2011, 111, 5215–5246; - PubMed
- Sun S; Fu J, Methyl-containing pharmaceuticals: Methylation in drug design. Bioorg. Med. Chem. Lett 2018, 28, 3283–3289. - PubMed
-
- Kariofillis SK; Shields BJ; Tekle-Smith MA; Zacuto MJ; Doyle AG, Nickel/Photoredox-Catalyzed Methylation of (Hetero)aryl Chlorides Using Trimethyl Orthoformate as a Methyl Radical Source. J. Am. Chem. Soc 2020, 142, 7683–7689; - PMC - PubMed
- Huihui KMM; Caputo JA; Melchor Z; Olivares AM; Spiewak AM; Johnson KA; DiBenedetto TA; Kim S; Ackerman LKG; Weix DJ, Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc 2016, 138, 5016–5019; - PMC - PubMed
- Zhang P; Le CC; MacMillan DWC, Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138, 8084–8087; - PMC - PubMed
- Wang J; Zhao J; Gong H, Nickel-catalyzed methylation of aryl halides/tosylates with methyl tosylate. Chem. Comm 2017, 53, 10180–10183; - PubMed
- Liang Z; Xue W; Lin K; Gong H, Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett 2014, 16, 5620–5623. - PubMed
-
- Dawson DD; Jarvo ER, Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers with Isotopically-Labeled Grignard Reagents. Org. Process Res. Dev 2015, 19, 1356–1359; - PMC - PubMed
- Taylor BLH; Swift EC; Waetzig JD; Jarvo ER, Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Alkyl Ethers: Enantioselective Synthesis of Diarylethanes. J. Am. Chem. Soc 2011, 133, 389–391; - PubMed
- Tollefson EJ; Dawson DD; Osborne CA; Jarvo ER, Stereospecific Cross-Coupling Reactions of Aryl-Substituted Tetrahydrofurans, Tetrahydropyrans, and Lactones. J. Am. Chem. Soc 2014, 136, 14951–14958; - PMC - PubMed
- Wisniewska HM; Swift EC; Jarvo ER, Functional-Group-Tolerant, Nickel-Catalyzed Cross-Coupling Reaction for Enantioselective Construction of Tertiary Methyl-Bearing Stereocenters. J. Am. Chem. Soc 2013, 135, 9083–9090; - PMC - PubMed
- Arp FO; Fu GC, Catalytic Enantioselective Negishi Reactions of Racemic Secondary Benzylic Halides. J. Am. Chem. Soc 2005, 127, 10482–10483; - PubMed
- Fischer C; Fu GC, Asymmetric Nickel-Catalyzed Negishi Cross-Couplings of Secondary α-Bromo Amides with Organozinc Reagents. J. Am. Chem. Soc 2005, 127, 4594–4595; - PubMed
- Tollefson EJ; Hanna LE; Jarvo ER, Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers and Esters. Acc. Chem. Res 2015, 48, 2344–2353; - PMC - PubMed
- Greene MA; Yonova IM; Williams FJ; Jarvo ER, Traceless Directing Group for Stereospecific Nickel-Catalyzed Alkyl−Alkyl Cross-Coupling Reactions. Org. Lett 2012, 14, 4293–4296; - PubMed
- Chen Y, Recent Advances in Methylation: A Guide for Selecting Methylation Reagents. Chem. - Eur. J 2019, 25, 3405–3439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

