Objective medication adherence and persistence in people with multiple sclerosis: a systematic review, meta-analysis, and meta-regression
- PMID: 34464209
- PMCID: PMC10391062
- DOI: 10.18553/jmcp.2021.27.9.1273
Objective medication adherence and persistence in people with multiple sclerosis: a systematic review, meta-analysis, and meta-regression
Abstract
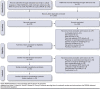
BACKGROUND: Medication adherence is critical for the realization of pharmacotherapy benefits and reduced healthcare expenditure. Studies have shown up to 60% of people with Multiple sclerosis (MS) experience suboptimal medication adherence, which is associated with poorer health outcomes and subsequent discontinuation. The current systematic review reported on objectively measured adherence and discontinuation rates for self-administered oral and injectable disease-modifying therapies (DMTs). OBJECTIVES: To identify whether, in people with MS, the introduction of oral DMTs has improved medication adherence when compared with injectable DMTs. The secondary aim was to report synthesized objectively measured medication adherence and persistence rates for both oral and injectable DMTs in MS across varying study durations. METHODS: Literature searches were conducted through PubMed, Web of Science, Scopus, and PsycINFO. Inclusion criteria were limited to English, peer-reviewed, objective, self-administered DMT articles, published between July 1993 to December 2019. Publications reporting combined intravenous and self-administered DMT data, or that did not account for DMT switching in discontinuation rates, were excluded. Data were synthesized into observation lengths ranging from less than 8 months to greater than 36 months. Meta-analysis and meta-regression were undertaken on both oral and injectable 12-month adherence and discontinuation data. RESULTS: In total, 61 articles were included; 46 articles examined adherence and 26 examined discontinuation. Twelve-month adherence ranged between 53.0% to 89.2% for oral (N = 7) and 47.0% to 77.4% for injectable DMTs (N = 7). Results from the meta-analysis and meta-regression show significantly higher pooled mean medication possession ratio (MPR) adherence for oral DMTs (91.0%) when compared to injectable DMTs (77.0%) over 12 months (β = -0.146; 95% CI: -0.263 to -0.029). Results indicate major asymmetry across studies (LFK index: -5.18), proposing the presence of significant publication bias. Mean discontinuation over 12 months was between 10.5% to 33.3% for oral (N = 7) and 15.2% to 50.8% for injectable DMTs (N = 10), with meta-analysis results indicating the presence of significant heterogeneity (I2 Injectable: 99.5%; I2 Oral: 93.1%) between studies included in each subgroup. However, no appreciable difference in mean discontinuation rates across groups (Injectable: 27%; 95% Cl: 19.0%-34.0%; Oral: 24%; 95% CI: 17.0%-31.0%) was found. CONCLUSIONS: Medication adherence for oral DMTs suggests a significant improvement compared to adherence for injectable DMTs. No significant difference in discontinuation rates between oral and injectable DMTs was found. Oral DMT adherence and persistence studies are limited, given their relatively recent introduction. Suboptimal medication adherence and discontinuation issues remain present for both oral and injectable DMTs. Future studies would benefit from improved consistency in methodology, such as comparable adherence and persistence definitions. DISCLOSURES: The authors did not receive any funding for this study. Mardan and Hussain have nothing to disclose. Grech reports grants from Merck Pharmaceutical, outside the submitted work. Allan reports grants received from Merck Pharmaceutical outside the submitted work. Allan holds advisory board and consulting positions with Merck and advisory board positions for Bristol Myers Squibb and Novartis, for which Monash Institute of Neurological Diseases receives consulting fees.
Conflict of interest statement
The authors did not receive any funding for this study. Mardan and Hussain have nothing to disclose. Grech reports grants from Merck Pharmaceutical, outside the submitted work. Allan reports grants received from Merck Pharmaceutical outside the submitted work. Allan holds advisory board and consulting positions with Merck and advisory board positions for Bristol Myers Squibb and Novartis, for which Monash Institute of Neurological Diseases receives consulting fees.
Figures
References
-
- Giovannoni G, Comi G, Cook S, et al. . A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. New Engl J Med. 2010;362(5):416-26. doi:10.1056/NEJMoa0902533 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical