Effect of Esophageal Pressure-guided Positive End-Expiratory Pressure on Survival from Acute Respiratory Distress Syndrome: A Risk-based and Mechanistic Reanalysis of the EPVent-2 Trial
- PMID: 34464237
- PMCID: PMC8759303
- DOI: 10.1164/rccm.202009-3539OC
Effect of Esophageal Pressure-guided Positive End-Expiratory Pressure on Survival from Acute Respiratory Distress Syndrome: A Risk-based and Mechanistic Reanalysis of the EPVent-2 Trial
Abstract
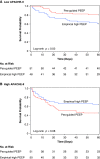
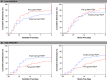
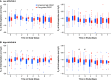
Rationale: In acute respiratory distress syndrome (ARDS), the effect of positive end-expiratory pressure (PEEP) may depend on the extent to which multiorgan dysfunction contributes to risk of death, and the precision with which PEEP is titrated to attenuate atelectrauma without exacerbating overdistension. Objectives: To evaluate whether multiorgan dysfunction and lung mechanics modified treatment effect in the EPVent-2 (Esophageal Pressure-guided Ventilation 2) trial, a multicenter trial of esophageal pressure (Pes)-guided PEEP versus empirical high PEEP in moderate to severe ARDS. Methods: This post hoc reanalysis of the EPVent-2 trial evaluated for heterogeneity of treatment effect on mortality by baseline multiorgan dysfunction, determined via Acute Physiology and Chronic Health Evaluation II (APACHE-II). It also evaluated whether PEEP titrated to end-expiratory transpulmonary pressure near 0 cm H2O was associated with survival. Measurements and Main Results: All 200 trial participants were included. Treatment effect on 60-day mortality differed by multiorgan dysfunction severity (P = 0.03 for interaction). Pes-guided PEEP was associated with lower mortality among patients with APACHE-II less than the median value (hazard ratio, 0.43; 95% confidence interval, 0.20-0.92) and may have had the opposite effect in patients with higher APACHE-II (hazard ratio, 1.69; 95% confidence interval, 0.93-3.05). Independent of treatment group or multiorgan dysfunction severity, mortality was lowest when PEEP titration achieved end-expiratory transpulmonary pressure near 0 cm H2O. Conclusions: The effect on survival of Pes-guided PEEP, compared with empirical high PEEP, differed by multiorgan dysfunction severity. Independent of multiorgan dysfunction, PEEP titrated to end-expiratory transpulmonary pressure closer to 0 cm H2O was associated with greater survival than more positive or negative values. These findings warrant prospective testing in a future trial.
Keywords: acute respiratory distress syndrome; mechanical ventilation; positive end-expiratory pressure; randomized controlled trial; ventilator-induced lung injury.
Figures



Comment in
-
Transpulmonary Pressure to Guide Mechanical Ventilation: Art or Science?Am J Respir Crit Care Med. 2021 Nov 15;204(10):1120-1121. doi: 10.1164/rccm.202109-2116ED. Am J Respir Crit Care Med. 2021. PMID: 34672875 Free PMC article. No abstract available.
References
-
- Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med . 2008;178:346–355. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

