Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19
- PMID: 34464358
- PMCID: PMC8516466
- DOI: 10.1172/JCI152264
Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19
Abstract
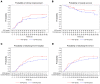
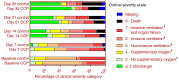
BACKGROUNDCOVID-19 convalescent plasma (CCP) has been considered a treatment option for COVID-19. This trial assessed the efficacy of a neutralizing antibody containing high-dose CCP in hospitalized adults with COVID-19 requiring respiratory support or intensive care treatment.METHODSPatients (n = 105) were randomized 1:1 to either receive standard treatment and 3 units of CCP or standard treatment alone. Control group patients with progress on day 14 could cross over to the CCP group. The primary outcome was a dichotomous composite outcome of survival and no longer fulfilling criteria for severe COVID-19 on day 21.ResultsThe primary outcome occurred in 43.4% of patients in the CCP group and 32.7% in the control group (P = 0.32). The median time to clinical improvement was 26 days in the CCP group and 66 days in the control group (P = 0.27). The median time to discharge from the hospital was 31 days in the CCP group and 51 days in the control group (P = 0.24). In the subgroup that received a higher cumulative amount of neutralizing antibodies, the primary outcome occurred in 56.0% of the patients (vs. 32.1%), with significantly shorter intervals to clinical improvement (20 vs. 66 days, P < 0.05) and to hospital discharge (21 vs. 51 days, P = 0.03) and better survival (day-60 probability of survival 91.6% vs. 68.1%, P = 0.02) in comparison with the control group.ConclusionCCP added to standard treatment was not associated with a significant improvement in the primary and secondary outcomes. A predefined subgroup analysis showed a significant benefit of CCP among patients who received a larger amount of neutralizing antibodies.Trial registrationClinicalTrials.gov NCT04433910.FundingBundesministerium für Gesundheit (German Federal Ministry of Health): ZMVI1-2520COR802.
Keywords: COVID-19; Clinical Trials; Immunoglobulins.
Conflict of interest statement
Figures

References
-
- US Food and Drug Administration. FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising Covid-19 Treatment, Another Achievement in Administration’s Fight Against Pandemic. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency... Updated August 23, 2020. Accessed August 31, 2021.
-
- Estcourt LJ, Roberts DJ. Convalescent plasma for Covid-19. BMJ. 2020;370:m3516. - PubMed

