Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study
- PMID: 34464382
- PMCID: PMC8407540
- DOI: 10.1371/journal.pmed.1003741
Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study
Abstract
Background: For locally advanced rectal cancer (LARC) patients who receive neoadjuvant chemoradiotherapy (nCRT), there are no reliable indicators to accurately predict pathological complete response (pCR) before surgery. For patients with clinical complete response (cCR), a "Watch and Wait" (W&W) approach can be adopted to improve quality of life. However, W&W approach may increase the recurrence risk in patients who are judged to be cCR but have minimal residual disease (MRD). Magnetic resonance imaging (MRI) is a major tool to evaluate response to nCRT; however, its ability to predict pCR needs to be improved. In this prospective cohort study, we explored the value of circulating tumor DNA (ctDNA) in combination with MRI in the prediction of pCR before surgery and investigated the utility of ctDNA in risk stratification and prognostic prediction for patients undergoing nCRT and total mesorectal excision (TME).
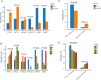
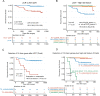
Methods and findings: We recruited 119 Chinese LARC patients (cT3-4/N0-2/M0; median age of 57; 85 males) who were treated with nCRT plus TME at Fudan University Shanghai Cancer Center (China) from February 7, 2016 to October 31, 2017. Plasma samples at baseline, during nCRT, and after surgery were collected. A total of 531 plasma samples were collected and subjected to deep targeted panel sequencing of 422 cancer-related genes. The association among ctDNA status, treatment response, and prognosis was analyzed. The performance of ctDNA alone, MRI alone, and combining ctDNA with MRI was evaluated for their ability to predict pCR/non-pCR. Ranging from complete tumor regression (pathological tumor regression grade 0; pTRG0) to poor regression (pTRG3), the ctDNA clearance rate during nCRT showed a significant decreasing trend (95.7%, 77.8%, 71.1%, and 66.7% in pTRG 0, 1, 2, and 3 groups, respectively, P = 0.008), while the detection rate of acquired mutations in ctDNA showed an increasing trend (3.8%, 8.3%, 19.2%, and 23.1% in pTRG 0, 1, 2, and 3 groups, respectively, P = 0.02). Univariable logistic regression showed that ctDNA clearance was associated with a low probability of non-pCR (odds ratio = 0.11, 95% confidence interval [95% CI] = 0.01 to 0.6, P = 0.04). A risk score predictive model, which incorporated both ctDNA (i.e., features of baseline ctDNA, ctDNA clearance, and acquired mutation status) and MRI tumor regression grade (mrTRG), was developed and demonstrated improved performance in predicting pCR/non-pCR (area under the curve [AUC] = 0.886, 95% CI = 0.810 to 0.962) compared with models derived from only ctDNA (AUC = 0.818, 95% CI = 0.725 to 0.912) or only mrTRG (AUC = 0.729, 95% CI = 0.641 to 0.816). The detection of potential colorectal cancer (CRC) driver genes in ctDNA after nCRT indicated a significantly worse recurrence-free survival (RFS) (hazard ratio [HR] = 9.29, 95% CI = 3.74 to 23.10, P < 0.001). Patients with detectable driver mutations and positive high-risk feature (HR_feature) after surgery had the highest recurrence risk (HR = 90.29, 95% CI = 17.01 to 479.26, P < 0.001). Limitations include relatively small sample size, lack of independent external validation, no serial ctDNA testing after surgery, and a relatively short follow-up period.
Conclusions: The model combining ctDNA and MRI improved the predictive performance compared with the models derived from individual information, and combining ctDNA with HR_feature can stratify patients with a high risk of recurrence. Therefore, ctDNA can supplement MRI to better predict nCRT response, and it could potentially help patient selection for nonoperative management and guide the treatment strategy for those with different recurrence risks.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: HB, XF, and XW are the employees of Geneseeq Technology Inc. YX, HB and YS are the employees of Nanjing Geneseeq Technology Inc.
Figures


References
-
- Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al.. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756 ; PubMed Central PMCID: PMC4666003. - DOI - PMC - PubMed
-
- Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al.. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

