What is the dynamical regime of cerebral cortex?
- PMID: 34464597
- PMCID: PMC9129095
- DOI: 10.1016/j.neuron.2021.07.031
What is the dynamical regime of cerebral cortex?
Abstract
Many studies have shown that the excitation and inhibition received by cortical neurons remain roughly balanced across many conditions. A key question for understanding the dynamical regime of cortex is the nature of this balancing. Theorists have shown that network dynamics can yield systematic cancellation of most of a neuron's excitatory input by inhibition. We review a wide range of evidence pointing to this cancellation occurring in a regime in which the balance is loose, meaning that the net input remaining after cancellation of excitation and inhibition is comparable in size with the factors that cancel, rather than tight, meaning that the net input is very small relative to the canceling factors. This choice of regime has important implications for cortical functional responses, as we describe: loose balance, but not tight balance, can yield many nonlinear population behaviors seen in sensory cortical neurons, allow the presence of correlated variability, and yield decrease of that variability with increasing external stimulus drive as observed across multiple cortical areas.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

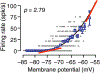
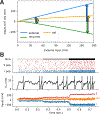

References
-
- Amit D. and Brunel N. (1997). Dynamics of a recurrent network of spiking neurons before and following learning. Network: Comput. Neural Syst, 8:373–404.
-
- Anderson JS, Carandini M, and Ferster D. (2000). Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol, 84:909–926. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

