Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome
- PMID: 34464739
- PMCID: PMC8863543
- DOI: 10.1016/j.neuroimage.2021.118530
Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome
Abstract
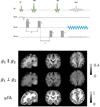
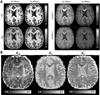
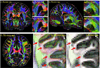
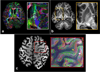
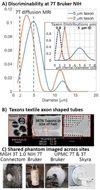
The first phase of the Human Connectome Project pioneered advances in MRI technology for mapping the macroscopic structural connections of the living human brain through the engineering of a whole-body human MRI scanner equipped with maximum gradient strength of 300 mT/m, the highest ever achieved for human imaging. While this instrument has made important contributions to the understanding of macroscale connectional topology, it has also demonstrated the potential of dedicated high-gradient performance scanners to provide unparalleled in vivo assessment of neural tissue microstructure. Building on the initial groundwork laid by the original Connectome scanner, we have now embarked on an international, multi-site effort to build the next-generation human 3T Connectome scanner (Connectome 2.0) optimized for the study of neural tissue microstructure and connectional anatomy across multiple length scales. In order to maximize the resolution of this in vivo microscope for studies of the living human brain, we will push the diffusion resolution limit to unprecedented levels by (1) nearly doubling the current maximum gradient strength from 300 mT/m to 500 mT/m and tripling the maximum slew rate from 200 T/m/s to 600 T/m/s through the design of a one-of-a-kind head gradient coil optimized to minimize peripheral nerve stimulation; (2) developing high-sensitivity multi-channel radiofrequency receive coils for in vivo and ex vivo human brain imaging; (3) incorporating dynamic field monitoring to minimize image distortions and artifacts; (4) developing new pulse sequences to integrate the strongest diffusion encoding and highest spatial resolution ever achieved in the living human brain; and (5) calibrating the measurements obtained from this next-generation instrument through systematic validation of diffusion microstructural metrics in high-fidelity phantoms and ex vivo brain tissue at progressively finer scales with accompanying diffusion simulations in histology-based micro-geometries. We envision creating the ultimate diffusion MRI instrument capable of capturing the complex multi-scale organization of the living human brain - from the microscopic scale needed to probe cellular geometry, heterogeneity and plasticity, to the mesoscopic scale for quantifying the distinctions in cortical structure and connectivity that define cyto- and myeloarchitectonic boundaries, to improvements in estimates of macroscopic connectivity.
Keywords: Axon diameter; Connectome; Diffusion MRI; Gray matter; Head gradient; Multi-scale modeling; Peripheral nerve stimulation; Tissue microstructure; Validation.
Copyright © 2021. Published by Elsevier Inc.
Figures





References
-
- Alexander DC, 2008. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magn. Reson. Med. 60, 439–448. - PubMed
-
- Alexander DC, Dyrby TB, Nilsson M, Zhang H, 2017. Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed.. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

