Enhanced Virus Detection and Metagenomic Sequencing in Patients with Meningitis and Encephalitis
- PMID: 34465023
- PMCID: PMC8406231
- DOI: 10.1128/mBio.01143-21
Enhanced Virus Detection and Metagenomic Sequencing in Patients with Meningitis and Encephalitis
Abstract
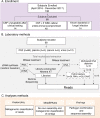
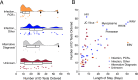
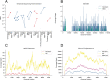
Meningitis and encephalitis are leading causes of central nervous system (CNS) disease and often result in severe neurological compromise or death. Traditional diagnostic workflows largely rely on pathogen-specific tests, sometimes over days to weeks, whereas metagenomic next-generation sequencing (mNGS) profiles all nucleic acid in a sample. In this single-center, prospective study, 68 hospitalized patients with known (n = 44) or suspected (n = 24) CNS infections underwent mNGS from RNA and DNA to identify potential pathogens and also targeted sequencing of viruses using hybrid capture. Using a computational metagenomic classification pipeline based on KrakenUniq and BLAST, we detected pathogen nucleic acid in cerebrospinal fluid (CSF) from 22 subjects, 3 of whom had no clinical diagnosis by routine workup. Among subjects diagnosed with infection by serology and/or peripheral samples, we demonstrated the utility of mNGS to detect pathogen nucleic acid in CSF, importantly for the Ixodes scapularis tick-borne pathogens Powassan virus, Borrelia burgdorferi, and Anaplasma phagocytophilum. We also evaluated two methods to enhance the detection of viral nucleic acid, hybrid capture and methylated DNA depletion. Hybrid capture nearly universally increased viral read recovery. Although results for methylated DNA depletion were mixed, it allowed the detection of varicella-zoster virus DNA in two samples that were negative by standard mNGS. Overall, mNGS is a promising approach that can test for multiple pathogens simultaneously, with efficacy similar to that of pathogen-specific tests, and can uncover geographically relevant infectious CNS disease, such as tick-borne infections in New England. With further laboratory and computational enhancements, mNGS may become a mainstay of workup for encephalitis and meningitis. IMPORTANCE Meningitis and encephalitis are leading global causes of central nervous system (CNS) disability and mortality. Current diagnostic workflows remain inefficient, requiring costly pathogen-specific assays and sometimes invasive surgical procedures. Despite intensive diagnostic efforts, 40 to 60% of people with meningitis or encephalitis have no clear cause of CNS disease identified. As diagnostic uncertainty often leads to costly inappropriate therapies, the need for novel pathogen detection methods is paramount. Metagenomic next-generation sequencing (mNGS) offers the unique opportunity to circumvent these challenges using unbiased laboratory and computational methods. Here, we performed comprehensive mNGS from 68 prospectively enrolled patients with known (n = 44) or suspected (n = 24) CNS viral infection from a single center in New England and evaluated enhanced methods to improve the detection of CNS pathogens, including those not traditionally identified in the CNS by nucleic acid detection. Overall, our work helps elucidate how mNGS can become integrated into the diagnostic toolkit for CNS infections.
Keywords: encephalitis; hybrid capture; meningitis; metagenomic sequencing; methylated DNA depletion; next-generation sequencing (NGS); virus.
Figures

Similar articles
-
Metagenomic Next-Generation Sequencing for Diagnosis of Infectious Encephalitis and Meningitis: A Large, Prospective Case Series of 213 Patients.Front Cell Infect Microbiol. 2020 Mar 5;10:88. doi: 10.3389/fcimb.2020.00088. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32211343 Free PMC article.
-
Evaluation of metagenomic and pathogen-targeted next-generation sequencing for diagnosis of meningitis and encephalitis in adults: A multicenter prospective observational cohort study in China.J Infect. 2024 May;88(5):106143. doi: 10.1016/j.jinf.2024.106143. Epub 2024 Mar 26. J Infect. 2024. PMID: 38548243
-
Integrating DNA/RNA microbe detection and host response for accurate diagnosis, treatment and prognosis of childhood infectious meningitis and encephalitis.J Transl Med. 2024 Jun 20;22(1):583. doi: 10.1186/s12967-024-05370-w. J Transl Med. 2024. PMID: 38902725 Free PMC article.
-
Clinical Metagenomic Next-Generation Sequencing for Diagnosis of Central Nervous System Infections: Advances and Challenges.Mol Diagn Ther. 2024 Sep;28(5):513-523. doi: 10.1007/s40291-024-00727-9. Epub 2024 Jul 11. Mol Diagn Ther. 2024. PMID: 38992308 Review.
-
Application of metagenomic next-generation sequencing in the diagnosis of infectious diseases.Front Cell Infect Microbiol. 2024 Nov 15;14:1458316. doi: 10.3389/fcimb.2024.1458316. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39619659 Free PMC article. Review.
Cited by
-
Viral meningoencephalitis in pediatric solid organ or hematopoietic cell transplant recipients: a diagnostic and therapeutic approach.Front Pediatr. 2024 Feb 12;12:1259088. doi: 10.3389/fped.2024.1259088. eCollection 2024. Front Pediatr. 2024. PMID: 38410764 Free PMC article. Review.
-
Using CSF Proteomics to Investigate Herpesvirus Infections of the Central Nervous System.Viruses. 2022 Dec 10;14(12):2757. doi: 10.3390/v14122757. Viruses. 2022. PMID: 36560759 Free PMC article.
-
Clinical Value of Metagenomic Next-Generation Sequencing in Immunocompromised Patients with Sepsis.Med Sci Monit. 2022 Aug 12;28:e937041. doi: 10.12659/MSM.937041. Med Sci Monit. 2022. PMID: 35957507 Free PMC article.
-
Metagenomic next-generation sequencing and proteomics analysis in pediatric viral encephalitis and meningitis.Front Cell Infect Microbiol. 2023 Apr 21;13:1104858. doi: 10.3389/fcimb.2023.1104858. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37153144 Free PMC article.
-
Borrelia miyamotoi Meningoencephalitis in an Immunocompetent Patient.Open Forum Infect Dis. 2022 Jun 13;9(7):ofac295. doi: 10.1093/ofid/ofac295. eCollection 2022 Jul. Open Forum Infect Dis. 2022. PMID: 35873293 Free PMC article.
References
-
- McGill F, Griffiths MJ, Bonnett LJ, Geretti AM, Michael BD, Beeching NJ, McKee D, Scarlett P, Hart IJ, Mutton KJ, Jung A, Adan G, Gummery A, Sulaiman WAW, Ennis K, Martin AP, Haycox A, Miller A, Solomon T, UK Meningitis Study Investigators . 2018. Incidence, aetiology, and sequelae of viral meningitis in UK adults: a multicentre prospective observational cohort study. Lancet Infect Dis 18:992–1003. doi:10.1016/S1473-3099(18)30245-7. - DOI - PMC - PubMed
-
- Quist-Paulsen E, Ormaasen V, Kran A-MB, Dunlop O, Ueland PM, Ueland T, Eikeland R, Aukrust P, Nordenmark TH. 2019. Encephalitis and aseptic meningitis: short-term and long-term outcome, quality of life and neuropsychological functioning. Sci Rep 9:16158. doi:10.1038/s41598-019-52570-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

