Unravelling the mystery of female meiotic drive: where we are
- PMID: 34465214
- PMCID: PMC8437031
- DOI: 10.1098/rsob.210074
Unravelling the mystery of female meiotic drive: where we are
Abstract
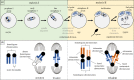
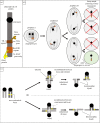
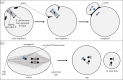
Female meiotic drive is the phenomenon where a selfish genetic element alters chromosome segregation during female meiosis to segregate to the egg and transmit to the next generation more frequently than Mendelian expectation. While several examples of female meiotic drive have been known for many decades, a molecular understanding of the underlying mechanisms has been elusive. Recent advances in this area in several model species prompts a comparative re-examination of these drive systems. In this review, we compare female meiotic drive of several animal and plant species, highlighting pertinent similarities.
Keywords: chromosome segregation; female meiosis; meiotic drive; selfish genetic elements.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Rice WR. 2013. Nothing in genetics makes sense except in light of genomic conflict. Annu. Rev. Ecol. Evol. Syst. 44, 217-237. (10.1146/annurev-ecolsys-110411-160242) - DOI
-
- Burt A, Trivers R. 2008. Genes in conflict: the biology of selfish genetic elements, pp. 325-380. Cambridge, MA: Belknap Press.
-
- Jones RN. 1991. B-chromosome drive. Am. Nat. 137, 430-442. (10.1086/285175) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

