Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates
- PMID: 34465872
- PMCID: PMC8770124
- DOI: 10.1038/s41390-021-01680-7
Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates
Abstract
Background: Necrotizing enterocolitis (NEC) is a major challenge for premature infants in neonatal intensive care units and efforts toward the search for indicators that could be used to predict the development of the disease have given limited results until now.
Methods: In this study, stools from 132 very low birth weight infants were collected daily in the context of a multi-center prospective study aimed at investigating the potential of fecal biomarkers for NEC prediction. Eight infants (~6%) received a stage 3 NEC diagnosis. Their stools collected up to 10 days before diagnosis were included and matched with 14 non-NEC controls and tested by ELISA for the quantitation of eight biomarkers.
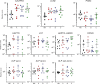
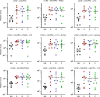
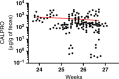
Results: Biomarkers were evaluated in all available stool samples leading to the identification of lipocalin-2 and calprotectin as the two most reliable predicting markers over the 10-day period prior to NEC development. Pooling the data for each infant confirmed the significance of lipocalin-2 and calprotectin, individually and in combination 1 week in advance of the NEC clinical diagnosis.
Conclusions: The lipocalin-2 and calprotectin tandem represents a significant biomarker signature for predicting NEC development. Although not yet fulfilling the "perfect biomarker" criteria, it represents a first step toward it.
Impact: Stool biomarkers can be used to predict NEC development in very low birth weight infants more than a week before the diagnosis. LCN2 was identified as a new robust biomarker for predicting NEC development, which used in conjunction with CALPRO, allows the identification of more than half of the cases that will develop NEC in very low birth weight infants. Combining more stool markers with the LCN2/CALPRO tandem such as PGE2 can further improve the algorithm for the prediction of NEC development.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Henry MC, Moss RL. Necrotizing enterocolitis. Annu. Rev. Med. 2009;60:111–124. - PubMed
-
- Heida FH, et al. Increased incidence of necrotizing enterocolitis in the Netherlands after implementation of the new Dutch guideline for active treatment in extremely preterm infants: results from three academic referral centers. J. Pediatr. Surg. 2017;52:273–276. - PubMed
-
- Fitzgibbons SC, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009;44:1072–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

