Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives
- PMID: 34466013
- PMCID: PMC8402953
- DOI: 10.2147/CEG.S293272
Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives
Abstract
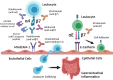
Leukocyte trafficking to the gastrointestinal tract is recognized to play a role in the pathogenesis of inflammatory bowel disease (IBD). Integrins are expressed on immune cells and interact with cell adhesion molecules (CAM) to mediate leukocyte trafficking. Blockade of the gut-tropic integrin α4β7 and its subunits has been exploited as a therapeutic target in IBD. Natalizumab (anti-α4) is approved for moderate to severe Crohn's disease (CD), but its use is limited due to potential risk of progressive multifocal leukoencephalopathy. Vedolizumab (anti-α4β7) is approved for the treatment of ulcerative colitis (UC) and CD. It is the most widely used anti-integrin therapy in IBD and has been shown to be effective in both induction and maintenance therapy, with a favorable safety profile. Several models incorporating clinical, genetic, immune, gut microbial, and vitamin D markers to predict response to vedolizumab in IBD have been developed. Etrolizumab (anti-β7) blocks leukocyte trafficking via α4β7 and cell adhesion via αEβ7 integrins. Large phase 3 clinical trials evaluating efficacy of etrolizumab in the induction and maintenance of patients with IBD are underway. Other investigational anti-integrin therapies include abrilumab (anti-α4β7 IgG2), PN-943 (orally administered and gut-restricted α4β7 antagonist peptide), AJM300 (orally active small molecule inhibitor of α4), and ontamalimab (anti-MAdCAM-1 IgG).
Keywords: Crohn’s disease; anti-integrin; etrolizumab; inflammatory bowel disease; natalizumab; ulcerative colitis; vedolizumab.
© 2021 Gubatan et al.
Conflict of interest statement
The authors have no conflicts of interests or financial disclosures relevant to this work.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources