Fusarium: more than a node or a foot-shaped basal cell
- PMID: 34466168
- PMCID: PMC8379525
- DOI: 10.1016/j.simyco.2021.100116
Fusarium: more than a node or a foot-shaped basal cell
Abstract
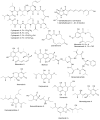
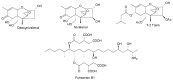
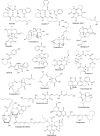
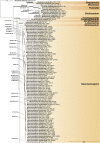
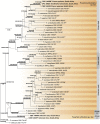
Recent publications have argued that there are potentially serious consequences for researchers in recognising distinct genera in the terminal fusarioid clade of the family Nectriaceae. Thus, an alternate hypothesis, namely a very broad concept of the genus Fusarium was proposed. In doing so, however, a significant body of data that supports distinct genera in Nectriaceae based on morphology, biology, and phylogeny is disregarded. A DNA phylogeny based on 19 orthologous protein-coding genes was presented to support a very broad concept of Fusarium at the F1 node in Nectriaceae. Here, we demonstrate that re-analyses of this dataset show that all 19 genes support the F3 node that represents Fusarium sensu stricto as defined by F. sambucinum (sexual morph synonym Gibberella pulicaris). The backbone of the phylogeny is resolved by the concatenated alignment, but only six of the 19 genes fully support the F1 node, representing the broad circumscription of Fusarium. Furthermore, a re-analysis of the concatenated dataset revealed alternate topologies in different phylogenetic algorithms, highlighting the deep divergence and unresolved placement of various Nectriaceae lineages proposed as members of Fusarium. Species of Fusarium s. str. are characterised by Gibberella sexual morphs, asexual morphs with thin- or thick-walled macroconidia that have variously shaped apical and basal cells, and trichothecene mycotoxin production, which separates them from other fusarioid genera. Here we show that the Wollenweber concept of Fusarium presently accounts for 20 segregate genera with clear-cut synapomorphic traits, and that fusarioid macroconidia represent a character that has been gained or lost multiple times throughout Nectriaceae. Thus, the very broad circumscription of Fusarium is blurry and without apparent synapomorphies, and does not include all genera with fusarium-like macroconidia, which are spread throughout Nectriaceae (e.g., Cosmosporella, Macroconia, Microcera). In this study four new genera are introduced, along with 18 new species and 16 new combinations. These names convey information about relationships, morphology, and ecological preference that would otherwise be lost in a broader definition of Fusarium. To assist users to correctly identify fusarioid genera and species, we introduce a new online identification database, Fusarioid-ID, accessible at www.fusarium.org. The database comprises partial sequences from multiple genes commonly used to identify fusarioid taxa (act1, CaM, his3, rpb1, rpb2, tef1, tub2, ITS, and LSU). In this paper, we also present a nomenclator of names that have been introduced in Fusarium up to January 2021 as well as their current status, types, and diagnostic DNA barcode data. In this study, researchers from 46 countries, representing taxonomists, plant pathologists, medical mycologists, quarantine officials, regulatory agencies, and students, strongly support the application and use of a more precisely delimited Fusarium (= Gibberella) concept to accommodate taxa from the robust monophyletic node F3 on the basis of a well-defined and unique combination of morphological and biochemical features. This F3 node includes, among others, species of the F. fujikuroi, F. incarnatum-equiseti, F. oxysporum, and F. sambucinum species complexes, but not species of Bisifusarium [F. dimerum species complex (SC)], Cyanonectria (F. buxicola SC), Geejayessia (F. staphyleae SC), Neocosmospora (F. solani SC) or Rectifusarium (F. ventricosum SC). The present study represents the first step to generating a new online monograph of Fusarium and allied fusarioid genera (www.fusarium.org).
Keywords: Apiognomonia platani (Lév.) L. Lombard; Atractium ciliatum Link; Atractium pallidum Bonord.; Calloria tremelloides (Grev.) L. Lombard; Cephalosporium sacchari E.J. Butler; Cosmosporella cavisperma (Corda) Sand.-Den., L. Lombard & Crous; Cylindrodendrum orthosporum (Sacc. & P. Syd.) L. Lombard; Dialonectria volutella (Ellis & Everh.) L. Lombard & Sand.-Den.; Fusarium aeruginosum Delacr.; Fusarium agaricorum Sarrazin; Fusarium albidoviolaceum Dasz.; Fusarium aleyrodis Petch; Fusarium amentorum Lacroix; Fusarium annuum Leonian; Fusarium arcuatum Berk. & M.A. Curtis; Fusarium aridum O.A. Pratt; Fusarium armeniacum (G.A. Forbes et al.) L.W. Burgess & Summerell; Fusarium arthrosporioides Sherb.; Fusarium asparagi Delacr.; Fusarium batatas Wollenw.; Fusarium biforme Sherb.; Fusarium buharicum Jacz. ex Babajan & Teterevn.-Babajan; Fusarium cactacearum Pasin. & Buzz.-Trav.; Fusarium cacti-maxonii Pasin. & Buzz.-Trav.; Fusarium caudatum Wollenw.; Fusarium cavispermum Corda; Fusarium cepae Hanzawa; Fusarium cesatii Rabenh.; Fusarium citriforme Jamal.; Fusarium citrinum Wollenw.; Fusarium citrulli Taubenh.; Fusarium clavatum Sherb.; Fusarium coccinellum Kalchbr.; Fusarium cromyophthoron Sideris; Fusarium cucurbitae Taubenh.; Fusarium cuneiforme Sherb.; Fusarium delacroixii Sacc.; Fusarium dimerum var. nectrioides Wollenw.; Fusarium echinatum Sand.-Den. & G.J. Marais; Fusarium epicoccum McAlpine; Fusarium eucheliae Sartory, R. Sartory & J. Mey.; Fusarium fissum Peyl; Fusarium flocciferum Corda; Fusarium gemmiperda Aderh.; Fusarium genevense Dasz.; Fusarium graminearum Schwabe; Fusarium graminum Corda; Fusarium heterosporioides Fautrey; Fusarium heterosporum Nees & T. Nees; Fusarium idahoanum O.A. Pratt; Fusarium juruanum Henn.; Fusarium lanceolatum O.A. Pratt; Fusarium lateritium Nees; Fusarium loncheceras Sideris; Fusarium longipes Wollenw. & Reinking; Fusarium lyarnte J.L. Walsh, Sangal., L.W. Burgess, E.C.Y. Liew & Summerell; Fusarium malvacearum Taubenh.; Fusarium martii f. phaseoli Burkh.; Fusarium muentzii Delacr.; Fusarium nigrum O.A. Pratt; Fusarium oxysporum var. asclerotium Sherb.; Fusarium palczewskii Jacz.; Fusarium palustre W.H. Elmer & Marra; Fusarium polymorphum Matr.; Fusarium poolense Taubenh.; Fusarium prieskaense G.J. Marais & Sand.-Den.; Fusarium prunorum McAlpine; Fusarium pusillum Wollenw.; Fusarium putrefaciens Osterw.; Fusarium redolens Wollenw.; Fusarium reticulatum Mont.; Fusarium rhizochromatistes Sideris; Fusarium rhizophilum Corda; Fusarium rhodellum McAlpine; Fusarium roesleri Thüm.; Fusarium rostratum Appel & Wollenw.; Fusarium rubiginosum Appel & Wollenw.; Fusarium rubrum Parav.; Fusarium samoense Gehrm.; Fusarium scirpi Lambotte & Fautrey; Fusarium secalis Jacz.; Fusarium spinaciae Hungerf.; Fusarium sporotrichioides Sherb.; Fusarium stercoris Fuckel; Fusarium stilboides Wollenw.; Fusarium stillatum De Not. ex Sacc.; Fusarium sublunatum Reinking; Fusarium succisae Schröt. ex Sacc.; Fusarium tabacivorum Delacr.; Fusarium trichothecioides Wollenw.; Fusarium tritici Liebman; Fusarium tuberivorum Wilcox & G.K. Link; Fusarium tumidum var. humi Reinking; Fusarium ustilaginis Kellerm. & Swingle; Fusarium viticola Thüm.; Fusarium werrikimbe J.L. Walsh, L.W. Burgess, E.C.Y. Liew & B.A. Summerell; Fusarium willkommii Lindau; Fusarium xylarioides Steyaert; Fusarium zygopetali Delacr.; Fusicolla meniscoidea L. Lombard & Sand.-Den.; Fusicolla quarantenae J.D.P. Bezerra, Sand.-Den., Crous & Souza-Motta; Fusicolla sporellula Sand.-Den. & L. Lombard; Fusisporium andropogonis Cooke ex Thüm.; Fusisporium anthophilum A. Braun; Fusisporium arundinis Corda; Fusisporium avenaceum Fr.; Fusisporium clypeaster Corda; Fusisporium culmorum Wm.G. Sm.; Fusisporium didymum Harting; Fusisporium elasticae Thüm.; Fusisporium episphaericum Cooke & Ellis; Fusisporium flavidum Bonord.; Fusisporium hordei Wm.G. Sm.; Fusisporium incarnatum Roberge ex Desm.; Fusisporium lolii Wm.G. Sm.; Fusisporium pandani Corda; Gibberella phyllostachydicola W. Yamam.; Hymenella aurea (Corda) L. Lombard; Hymenella spermogoniopsis (Jul. Müll.) L. Lombard & Sand.-Den.; Luteonectria Sand.-Den., L. Lombard, Schroers & Rossman; Luteonectria albida (Rossman) Sand.-Den. & L. Lombard; Luteonectria nematophila (Nirenberg & Hagedorn) Sand.-Den. & L. Lombard; Macroconia bulbipes Crous & Sand.-Den.; Macroconia phlogioides Sand.-Den. & Crous; Menispora penicillata Harz; Multi-gene phylogeny; Mycotoxins; Nectriaceae; Neocosmospora; Neocosmospora epipeda Quaedvl. & Sand.-Den.; Neocosmospora floridana (T. Aoki et al.) L. Lombard & Sand.-Den.; Neocosmospora merkxiana Quaedvl. & Sand.-Den.; Neocosmospora neerlandica Crous & Sand.-Den.; Neocosmospora nelsonii Crous & Sand.-Den.; Neocosmospora obliquiseptata (T. Aoki et al.) L. Lombard & Sand.-Den.; Neocosmospora pseudopisi Sand.-Den. & L. Lombard; Neocosmospora rekana (Lynn & Marinc.) L. Lombard & Sand.-Den.; Neocosmospora tuaranensis (T. Aoki et al.) L. Lombard & Sand.-Den.; Nothofusarium Crous, Sand.-Den. & L. Lombard; Nothofusarium devonianum L. Lombard, Crous & Sand.-Den.; Novel taxa; Pathogen; Scolecofusarium L. Lombard, Sand.-Den. & Crous; Scolecofusarium ciliatum (Link) L. Lombard, Sand.-Den. & Crous; Selenosporium equiseti Corda; Selenosporium hippocastani Corda; Selenosporium sarcochroum Desm; Selenosporium urticearum Corda.; Setofusarium (Nirenberg & Samuels) Crous & Sand.-Den.; Setofusarium setosum (Samuels & Nirenberg) Sand.-Den. & Crous.; Sphaeria sanguinea var. cicatricum Berk.; Sporotrichum poae Peck.; Stylonectria corniculata Gräfenhan, Crous & Sand.-Den.; Stylonectria hetmanica Akulov, Crous & Sand.-Den.; Taxonomy.
© 2021 Westerdijk Fungal Biodiversity Institute. Production and hosting by ELSEVIER B.V.
Figures




















































References
-
- Abraham W.R., Hanssen H.P. Fusoxysporone-a new type of diterpene from Fusarium oxysporum. Tetrahedron. 1992;48:10559–10562.
-
- Al-Hatmi A.M.S., Ahmed S.A., van Diepeningen A.D. Fusarium metavorans sp. nov.: The frequent opportunist ‘FSSC6’. Medical Mycology. 2018;56:S144–S152. - PubMed
-
- Al-Hatmi A.M.S., Mirabolfathy M., Hagen F. DNA barcoding, MALDI-TOF, and AFLP data support Fusarium ficicrescens as a distinct species within the Fusarium fujikuroi species complex. Fungal Biology. 2016;120:265–278. - PubMed
-
- Al-Hatmi A.M.S., Normand A.C., van Diepeningen A.D. Rapid identification of clinical members of Fusarium fujikuroi complex using MALDI-TOF MS. Future Microbiology. 2015;10:1939–1952. - PubMed
