Cyclooxygenase-2 regulates PTHrP transcription in human articular chondrocytes and is involved in the pathophysiology of osteoarthritis in rats
- PMID: 34466385
- PMCID: PMC8374645
- DOI: 10.1016/j.jot.2021.06.003
Cyclooxygenase-2 regulates PTHrP transcription in human articular chondrocytes and is involved in the pathophysiology of osteoarthritis in rats
Abstract
Background: Cyclooxygenase-2 (COX-2) inhibitors are prescribed for the management of osteoarthritis (OA)-associated pain and inflammation. However, the role of COX-2 in normal and osteoarthritic articular chondrocytes has not been well investigated. We hypothesize that COX-2 plays a role in articular chondrocytes under normal conditions and during OA progression.
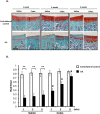
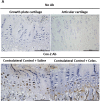
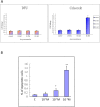
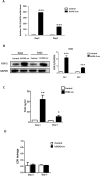
Methods: In vivo COX-2 levels in articular cartilage of normal and papain-induced osteoarthritic rats were compared. The role of COX-2 in human articular chondrocytes (HACs) was tested in vitro by COX-2 overexpression or activity inhibition. The levels of COX-2 and marker gene for normal function or articular cartilage degeneration were evaluated: mRNA by qRT-PCR; proteins by western blotting or immunohistochemistry; and glycosaminoglycan (GAG) by Safranin O-fast green staining. Parathyroid hormone-related protein (PTHrP) promoter activity was detected with luciferase reporter assays.
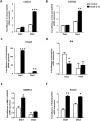
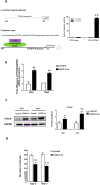
Results: In the OA rat study, COX-2 and PTHrP were simultaneously increased in osteoarthritic rat chondrocytes, while increased PTHrP levels were reduced by celecoxib, a COX-2 selective inhibitor. The levels of normal cartilage matrices, GAG and type II collagen decreased, while markers of degeneration, collagen type X and MMP13 were elevated in osteoarthritic articular chondrocytes. Celecoxib rescued the loss of GAG and the increased collagen type X and MMP13 levels. In vitro, COX-2 overexpression in HACs significantly increased Col2a1, Col10a1, PTHrP and MMP13 mRNA expression, which was decreased when COX-2 activity was suppressed. More importantly, COX-2 overexpression upregulated the PTHrP transcription, mRNA expression and protein levels.
Conclusion: COX-2 plays a pathophysiological role by preventing terminal differentiation of articular chondrocytes by upregulating PTHrP expression at the early stage of OA progression.
The translational potential of this article: COX2 up-regulates PTHrP expression in normal and OA articular chondrocytes.
Keywords: Articular chondrocytes; Cyclooxygenase-2 (COX-2); Osteoarthritis (OA); PTHrP; Terminal differentiation.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lanza F.L., Chan F.K., Quigley E.M. Practice parameters committee of the American college of G. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. - PubMed
-
- Laine L., White W.B., Rostom A., Hochberg M. COX-2 selective inhibitors in the treatment of osteoarthritis. Semin Arthritis Rheum. 2008;38(3):165–187. - PubMed
-
- Nakata K., Hanai T., Take Y., Osada T., Tsuchiya T., Shima D. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2018;26(10):1263–1273. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

