Persistent neuromuscular junction transmission defects in adults with spinal muscular atrophy treated with nusinersen
- PMID: 34466806
- PMCID: PMC8362737
- DOI: 10.1136/bmjno-2021-000164
Persistent neuromuscular junction transmission defects in adults with spinal muscular atrophy treated with nusinersen
Abstract
Objective: Spinal muscular atrophy (SMA) is a motor neuron disease caused by low levels of survival motor neuron (SMN) protein. Prior work in models and patients has demonstrated electrophysiological and morphological defects at the neuromuscular junction (NMJ). Therapeutic development has resulted in clinically available therapies to increase SMN protein levels in patients and improve muscle function. Here we aimed to investigate the effect of SMN restoration (via nusinersen) on NMJ transmission in adults with SMA.
Methods: Participants undergoing nusinersen treatment underwent 3 Hz repetitive nerve stimulation (RNS) of the spinal accessory nerve to assess compound muscle action potential amplitude decrement. Maximum voluntary isometric contraction (MVICT), Revised Upper Limb Module (RULM), and 6 min walk test (6MWT) were assessed for correlations with decrement.
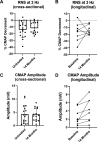
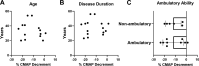
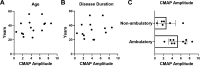
Results: Data from 13 ambulatory (7 men/6 women, mean age 40±11 years) and 11 non-ambulatory (3 men/8 women, mean age 38±12 years) participants were analysed. Cross-sectional analyses of RNS decrement were similar at 14 months of nusinersen (-14.2%±11.5%, n=17) vs baseline (-11.9%±8.3%, n=15) (unpaired t-test, p=0.5202). Longitudinal comparison of decrement in eight participants showed no change at 14 months (-13.9%±6.7%) vs baseline (-16.9%±13.4%) (paired t-test, p=0.5863). Decrement showed strong correlations with measures of MVICT, RULM and 6MWT but not age or disease duration.
Conclusion: Adults with SMA had significant NMJ transmission defects that were not corrected with 14 months of nusinersen treatment. NMJ defects were negatively associated with physical function, and thus may represent a promising target for additive or combinatorial treatments.
Keywords: EMG; neuromuscular; spinal muscular atrophy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BHE received compensation for consulting from Biogen, Genentech, Argenx and Stealth Bio-therapeutics. TW received compensation for consulting from Medtronic, Inc, and PainTeq. SK received compensation for consulting from Genentech, AveXis and Biogen. WDA received compensation for consulting for La Hoffmann Roche, Cadent Therapeutics, Novartis and Genentech. AHMB received compensation from AveXis for consulting. All the other authors report no disclosures.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
