A tissue culture infectious dose-derived protocol for testing of SARS-CoV-2 neutralization of serum antibodies on adherent cells
- PMID: 34467223
- PMCID: PMC8390364
- DOI: 10.1016/j.xpro.2021.100824
A tissue culture infectious dose-derived protocol for testing of SARS-CoV-2 neutralization of serum antibodies on adherent cells
Abstract
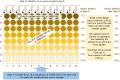
For a cytopathic virus such as severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), the neutralization capacity of serum from convalescent or vaccinated persons or of therapeutic antibodies can be tested on adherent cell cultures. Here, a simple and tissue culture infectious dose-derived protocol for assessment of neutralization of SARS-CoV-2 is described. Compared with the often applied plaque-forming unit assay, the working load is lower, and fewer manipulations of the infected cultures are required. Hence, the method is safer for the personnel.
Keywords: Cell culture; Cell-based Assays; Immunology; Microbiology.
© 2021 The Author(s).
Conflict of interest statement
T.M.K. is a medical advisor to Saiba Biotech AG (Pfaeffikon, Switzerland), which develops vaccines, also against SARS-CoV-2. P.J. has received financial support from Saiba Biotech AG, PCI Biotech (Oslo, Norway), and Allergy Therapeutics (Worthing, United Kingdom). The other authors declare no competing interests.
Figures






References
-
- Chu H., Chan J.F.-W., Yuen T.T.-T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C.-Y., Tsang J.O.-L., Huang X. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. - PMC - PubMed
-
- Thao T.T.N., Labroussaa F., Ebert N., V’kovski P., Stalder H., Portmann J., Kelly J., Steiner S., Holwerda M., Kratzel A. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582:561–565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

