Broadly Neutralizing Synthetic Cannabinoid Vaccines
- PMID: 34467269
- PMCID: PMC8395583
- DOI: 10.1021/jacsau.0c00057
Broadly Neutralizing Synthetic Cannabinoid Vaccines
Abstract
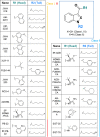
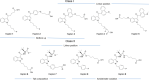
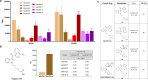
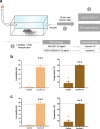
Synthetic cannabinoids (SCs) constitute a significant portion of psychoactive substances forming a major public health risk. Due to the wide variety of SCs, broadly neutralizing antibodies generated by active immunization present an intriguing pathway to combat cannabinoid use disorder. Here, we probed hapten design for antibody affinity and cross reactivity against two classes of SCs. Of the 10 haptens screened, 3 vaccine groups revealed submicromolar IC50, each targeting 5-6 compounds in our panel of 22 drugs. Moreover, SCs were successfully sequestered when administered by vaping or intraperitoneal injection, which was confirmed within animal models by observing locomotion, body temperature, and pharmacokinetics. We also discovered synergistic effects to simultaneously blunt two drug classes through an admixture vaccine approach. Collectively, our study provides a comprehensive foundation for the development of vaccines against SCs.
© 2020 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- World Drug Report 2019, Sales No. E.19.XI.8; United Nations publication, 2019.
-
- Evans-Brown M.; Gallegos A.; Christie R.. Fentanils and synthetic cannabinoids: driving greater complexity into the drug situation. An update from the EU Early Warning System; Publications Office of the European Union, 2018.
-
- Gummin D. D.; Mowry J. B.; Spyker D. A.; Brooks D. E.; Beuhler M. C.; Rivers L. J.; Hashem H. A.; Ryan M. L. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin. Toxicol. 2019, 57 (12), 1220–1413. 10.1080/15563650.2019.1677022. - DOI - PubMed
LinkOut - more resources
Full Text Sources
