Silica-Supported PdGa Nanoparticles: Metal Synergy for Highly Active and Selective CO2-to-CH3OH Hydrogenation
- PMID: 34467307
- PMCID: PMC8395611
- DOI: 10.1021/jacsau.1c00021
Silica-Supported PdGa Nanoparticles: Metal Synergy for Highly Active and Selective CO2-to-CH3OH Hydrogenation
Erratum in
-
Correction to "Silica-Supported PdGa Nanoparticles: Metal Synergy for Highly Active and Selective CO2-to-CH3OH Hydrogenation".JACS Au. 2022 Aug 12;2(8):1946-1947. doi: 10.1021/jacsau.2c00421. eCollection 2022 Aug 22. JACS Au. 2022. PMID: 36032539 Free PMC article.
Abstract
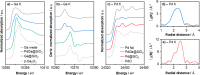
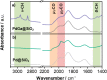
The direct conversion of CO2 to CH3OH represents an appealing strategy for the mitigation of anthropogenic CO2 emissions. Here, we report that small, narrowly distributed alloyed PdGa nanoparticles, prepared via surface organometallic chemistry from silica-supported GaIII isolated sites, selectively catalyze the hydrogenation of CO2 to CH3OH. At 230 °C and 25 bar, high activity (22.3 molMeOH molPd -1 h-1) and selectivity for CH3OH/DME (81%) are observed, while the corresponding silica-supported Pd nanoparticles show low activity and selectivity. X-ray absorption spectroscopy (XAS), IR, NMR, and scanning transmission electron microscopy-energy-dispersive X-ray provide evidence for alloying in the as-synthesized material. In situ XAS reveals that there is a dynamic dealloying/realloying process, through Ga redox, while operando diffuse reflectance infrared Fourier transform spectroscopy demonstrates that, while both methoxy and formate species are observed in reaction conditions, the relative concentrations are inversely proportional, as the chemical potential of the gas phase is modulated. High CH3OH selectivities, across a broad range of conversions, are observed, showing that CO formation is suppressed for this catalyst, in contrast to reported Pd catalysts.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Kondratenko E. V.; Mul G.; Baltrusaitis J.; Larrazábal G. O.; Pérez-Ramírez J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci. 2013, 6 (11), 3112–3135. 10.1039/c3ee41272e. - DOI
-
- Álvarez A.; Bansode A.; Urakawa A.; Bavykina A. V.; Wezendonk T. A.; Makkee M.; Gascon J.; Kapteijn F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117 (14), 9804–9838. 10.1021/acs.chemrev.6b00816. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
