From Chemical Curiosities and Trophy Molecules to Uranium-Based Catalysis: Developments for Uranium Catalysis as a New Facet in Molecular Uranium Chemistry
- PMID: 34467327
- PMCID: PMC8395704
- DOI: 10.1021/jacsau.1c00082
From Chemical Curiosities and Trophy Molecules to Uranium-Based Catalysis: Developments for Uranium Catalysis as a New Facet in Molecular Uranium Chemistry
Abstract
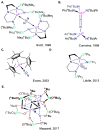
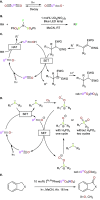
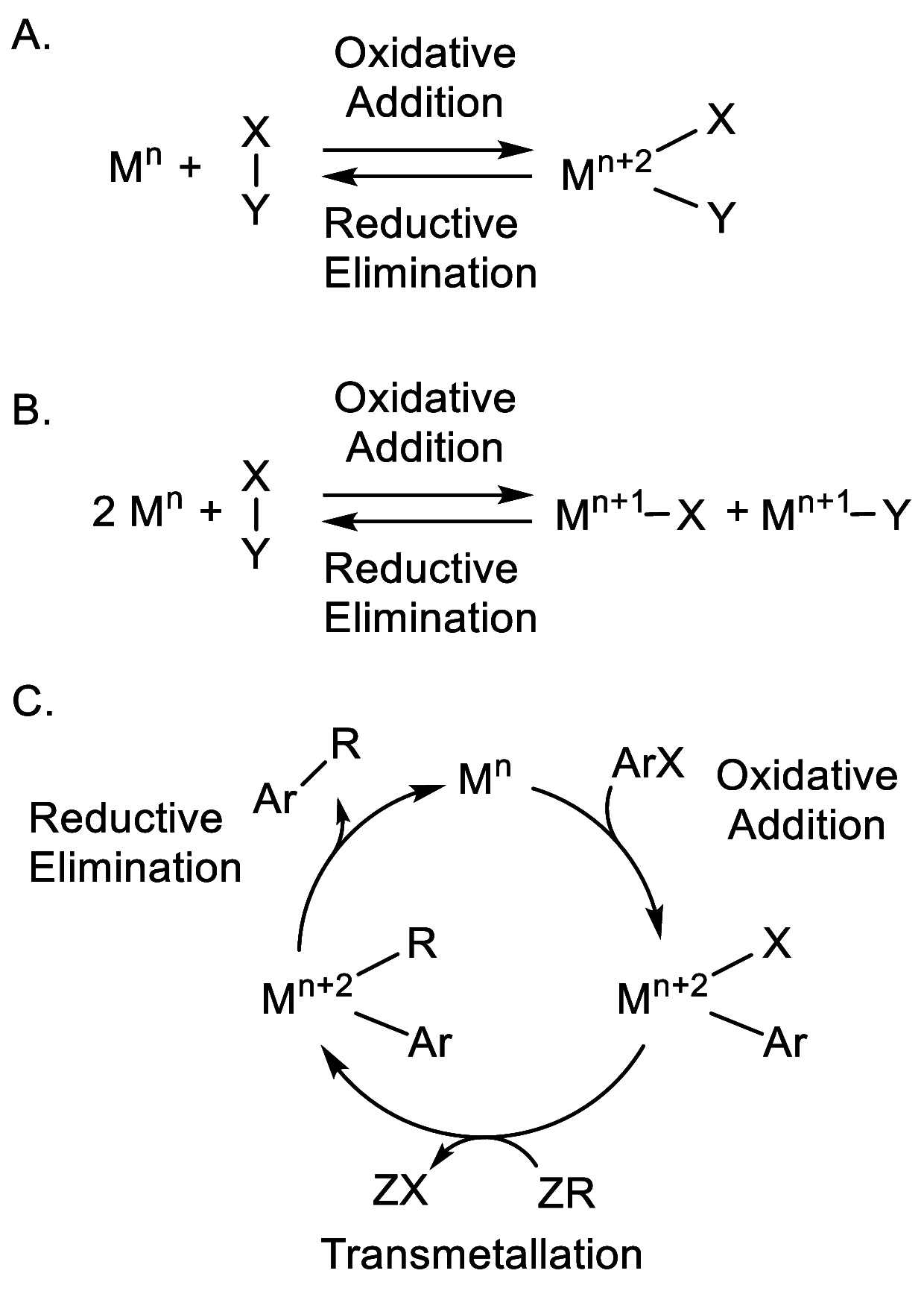
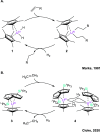
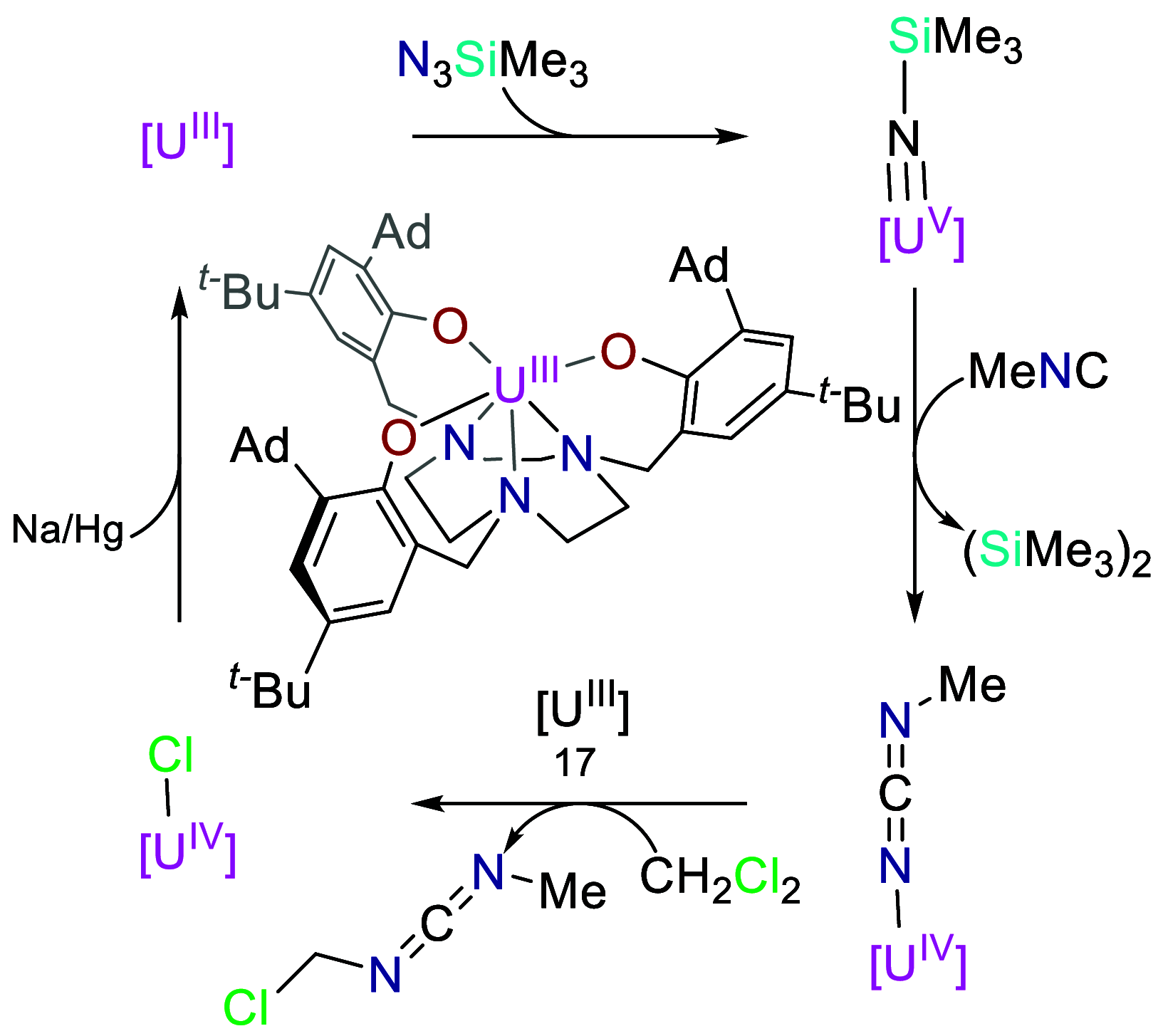
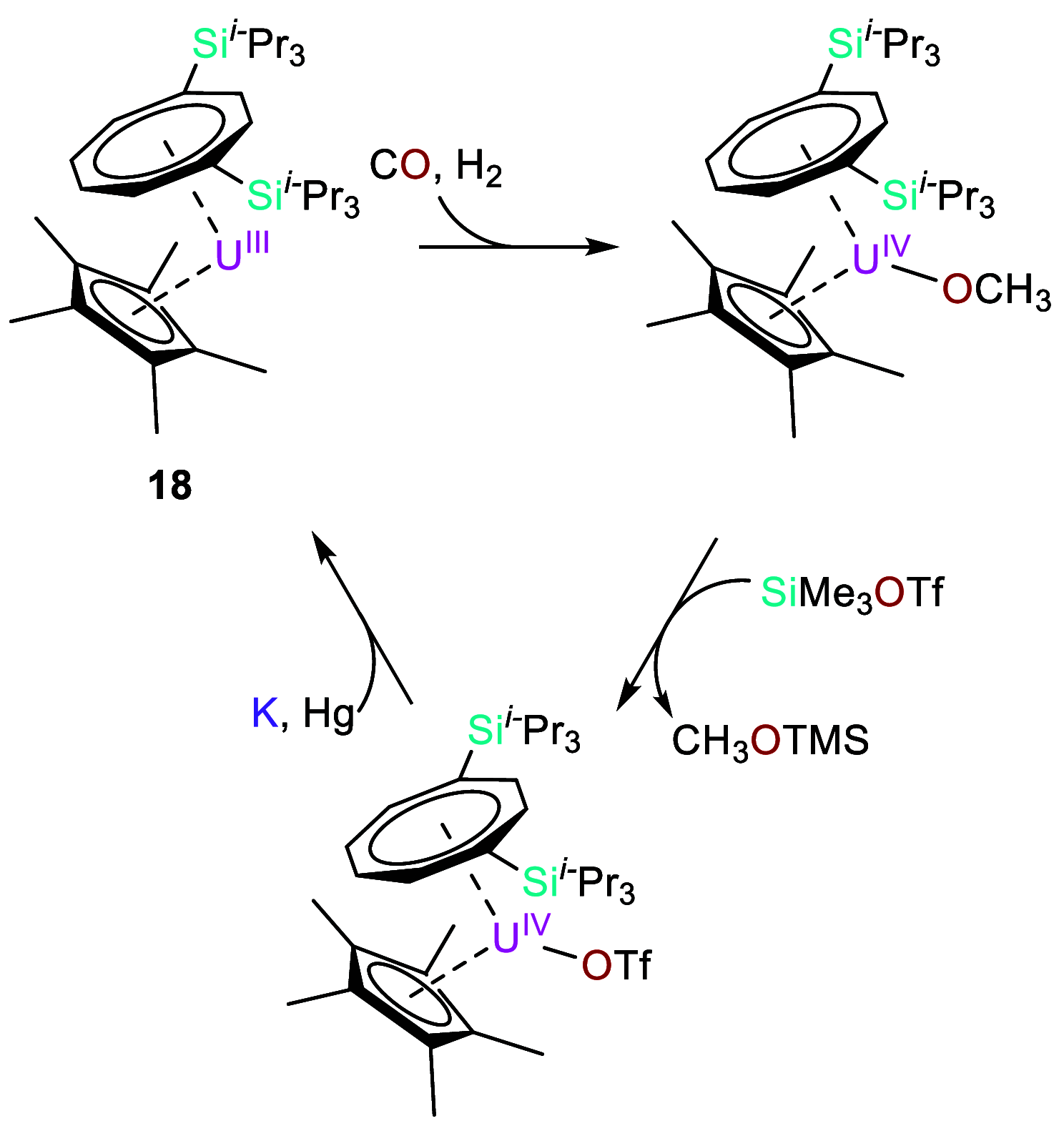
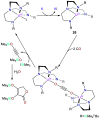
Catalysis remains one of the final frontiers in molecular uranium chemistry. Depleted uranium is mildly radioactive, continuously generated in large quantities from the production and consumption of nuclear fuels and accessible through the regeneration of "uranium waste". Organometallic complexes of uranium possess a number of properties that are appealing for applications in homogeneous catalysis. Uranium exists in a wide range of oxidation states, and its large ionic radii support chelating ligands with high coordination numbers resulting in increased complex stability. Its position within the actinide series allows it to involve its f-orbitals in partial covalent bonding; yet, the U-L bonds remain highly polarized. This causes these bonds to be reactive and, with few exceptions, relatively weak, allowing for high substrate on/off rates. Thus, it is reasonable that uranium could be considered as a source of metal catalysts. Accordingly, uranium complexes in oxidation states +4, +5, and +6 have been studied extensively as catalysts in sigma-bond metathesis reactions, with a body of literature spanning the past 40 years. High-valent species have been documented to perform a wide variety of reactions, including oligomerization, hydrogenation, and hydrosilylation. Concurrently, electron-rich uranium complexes in oxidation states +2 and +3 have been proven capable of performing reductive small molecule activation of N2, CO2, CO, and H2O. Hence, uranium's ability to activate small molecules of biological and industrial relevance is particularly pertinent when looking toward a sustainable future, especially due to its promising ability to generate ammonia, molecular hydrogen, and liquid hydrocarbons, though the advance of catalysis in these areas is in the early stages of development. In this Perspective, we will look at the challenges associated with the advance of new uranium catalysts, the tools produced to combat these challenges, the triumphs in achieving uranium catalysis, and our future outlook on the topic.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


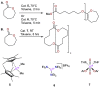
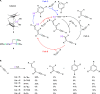
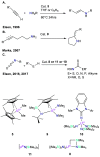

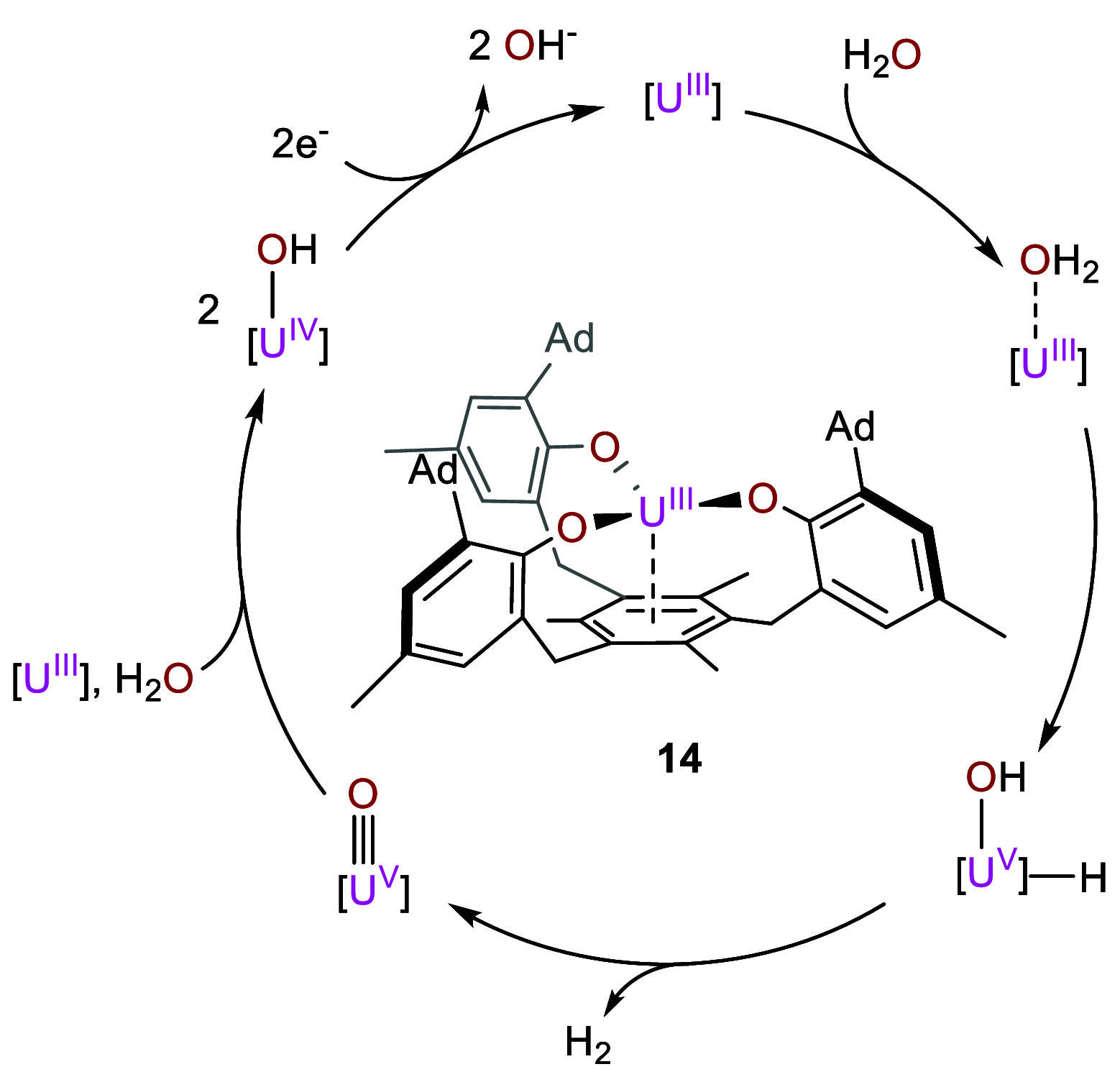

References
-
- Löffler S. T.; Meyer K.. Actinides. In Comprehensive Coordination Chemistry; Elsevier, Amsterdam, The Netherlands, 2019.10.1016/B978-0-12-409547-2.14754-7 - DOI
-
- Roussel P.; Scott P. Complex of Dinitrogen with Trivalent Uranium. J. Am. Chem. Soc. 1998, 120, 1070–1071. 10.1021/ja972933+. - DOI
-
- Odom A. L.; Arnold P. L.; Cummins C. C. Heterodinuclear Uranium/Molybdenum Dinitrogen Complexes. J. Am. Chem. Soc. 1998, 120, 5836–5837. 10.1021/ja980095t. - DOI
-
- Haber F.Verfahren Zur Herstellung von Ammoniak Durch Katalytische Vereinigung von Stickstoff Und Wasserstoff, Zweckmäßig Unter Hohem Druch. DE229126, 1909.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
