Thiol-Mediated Uptake
- PMID: 34467328
- PMCID: PMC8395643
- DOI: 10.1021/jacsau.1c00128
Thiol-Mediated Uptake
Abstract
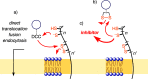
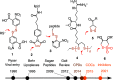
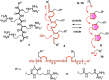
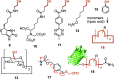
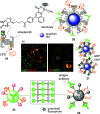
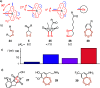
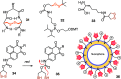
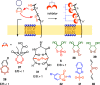
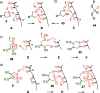
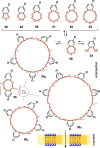
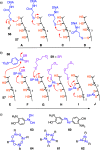
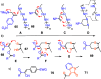
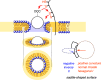
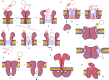
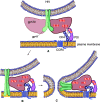
This Perspective focuses on thiol-mediated uptake, that is, the entry of substrates into cells enabled by oligochalcogenides or mimics, often disulfides, and inhibited by thiol-reactive agents. A short chronology from the initial observations in 1990 until today is followed by a summary of cell-penetrating poly(disulfide)s (CPDs) and cyclic oligochalcogenides (COCs) as privileged scaffolds in thiol-mediated uptake and inhibitors of thiol-mediated uptake as potential antivirals. In the spirit of a Perspective, the main part brings together topics that possibly could help to explain how thiol-mediated uptake really works. Extreme sulfur chemistry mostly related to COCs and their mimics, cyclic disulfides, thiosulfinates/-onates, diselenolanes, benzopolysulfanes, but also arsenics and Michael acceptors, is viewed in the context of acidity, ring tension, exchange cascades, adaptive networks, exchange affinity columns, molecular walkers, ring-opening polymerizations, and templated polymerizations. Micellar pores (or lipid ion channels) are considered, from cell-penetrating peptides and natural antibiotics to voltage sensors, and a concise gallery of membrane proteins, as possible targets of thiol-mediated uptake, is provided, including CLIC1, a thiol-reactive chloride channel; TMEM16F, a Ca-activated scramblase; EGFR, the epithelial growth factor receptor; and protein-disulfide isomerase, known from HIV entry or the transferrin receptor, a top hit in proteomics and recently identified in the cellular entry of SARS-CoV-2.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A U.S. Patent Application has been filed covering the antiviral activity of cyclic thiosulfonates (No. 63/073,863).
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
