Attitudes and behaviour regarding dose reduction of biologics for psoriasis: a survey among dermatologists worldwide
- PMID: 34467442
- PMCID: PMC9307528
- DOI: 10.1007/s00403-021-02273-4
Attitudes and behaviour regarding dose reduction of biologics for psoriasis: a survey among dermatologists worldwide
Abstract
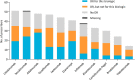
Dose reduction (DR) of biologics, where possible, seems promising for more efficient use of expensive biologics. For implementation of DR strategies, it is essential to get insight in factors that influence implementation. The objective of this study was to evaluate the attitudes and behaviour regarding dose reduction of biologic therapies for psoriasis among psoriasis expert dermatologists worldwide. A 27-question e-survey was sent through the International Psoriasis Council (IPC) to its 114 dermatologist councilors worldwide. The survey assessed demographics, general and DR prescription behaviour, and motivations for and barriers against application of DR. Of 57 respondents, 53 respondents who prescribed biologics were included for analysis. Thirty-seven (69.8%) applied DR (i.e., 'DR dermatologists'), and 16 (30.2%) did not (i.e., 'Non-DR dermatologists'). DR strategies varied among respondents. Regarding criteria for starting DR, differences were reported in required treatment duration, and interpretation and duration of stable low disease activity. In addition, the prolongation of intervals between injections varied between respondents. For most 'DR dermatologists' (n = 32/37, 86.5%), cost savings were one of the main reasons to apply DR. Fifteen out of 16 'Non-DR dermatologists' (94%) did not apply DR due to lack of scientific evidence. In conclusion, DR of biologics for psoriasis is part of clinical practice in psoriasis experts globally. Barriers for applying DR included lack of evidence or guidelines, and uncertainty on DR effects and risks. Although growing evidence shows DR feasibility, future studies are needed to accumulate and broaden evidence, along with development of (inter)national guidelines on DR strategies.
Keywords: Biologics; Dermatologists; Dose reduction; Psoriasis; Survey.
© 2021. The Author(s).
Conflict of interest statement
ME van Muijen carries out clinical trials for Abbvie, Celgene, Janssen and Novartis, and has received speaking fees from Janssen. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands. LS van der Schoot carries out clinical trials for Janssen and Novartis. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands. JMPA van den Reek carries out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma and Eli Lilly and reimbursement for attending symposiums from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands. EMGJ de Jong has received research grants for the independent research fund of the department of dermatology of the Radboud University Medical Center Nijmegen, The Netherlands from AbbVie, Pfizer, Novartis, Janssen Pharmaceuticals and Leo Pharma and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen Pharmaceutica, Novartis, Lily, Celgene, Leo Pharma, UCB and Almirall. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands.
Figures
References
-
- Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, Callis Duffin K, Eichenfield LF, Garg A, Gelfand JM, Gottlieb AB, Koo JY, Korman NJ, Krueger GG, Lebwohl MG, Leonardi CL, Mandelin AM, Menter MA, Merola JF, Pariser DM, Prussick RB, Ryan C, Shah KN, Weinberg JM, Williams MO, Wu JJ, Yamauchi PS, Van Voorhees AS. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290–298. doi: 10.1016/j.jaad.2016.10.017. - DOI - PubMed
-
- Atalay S, van den Reek J, den Broeder AA, van Vugt LJ, Otero ME, Njoo MD, Mommers JM, Ossenkoppele PM, Koetsier MI, Berends MA, van de Kerkhof PCM, Groenewoud HMM, Kievit W, de Jong E. Comparison of tightly controlled dose reduction of biologics with usual care for patients with psoriasis: a randomized clinical trial. JAMA Dermatol. 2020;156:393–400. doi: 10.1001/jamadermatol.2019.4897. - DOI - PMC - PubMed
-
- Blauvelt A, Ferris LK, Yamauchi PS, Qureshi A, Leonardi CL, Farahi K, Fakharzadeh S, Hsu MC, Li S, Chevrier M, Smith K, Goyal K, Chen Y, Munoz-Elias EJ, Callis Duffin K. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR) Br J Dermatol. 2017;177:1552–1561. doi: 10.1111/bjd.15722. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

