Oxytocin antagonists for assisted reproduction
- PMID: 34467530
- PMCID: PMC8408576
- DOI: 10.1002/14651858.CD012375.pub2
Oxytocin antagonists for assisted reproduction
Abstract
Background: Embryo transfer (ET) is a crucial step of in vitro fertilisation (IVF) treatment, and involves placing the embryo(s) in the woman's uterus. There is a negative association between endometrial wave-like activity (contractile activities) at the time of ET and clinical pregnancy, but no specific treatment is currently used in clinical practice to counteract their effects. Oxytocin is a hormone produced by the hypothalamus and released by the posterior pituitary. Its main role involves generating uterine contractions during and after childbirth. Atosiban is the best known oxytocin antagonist (and is also a vasopressin antagonist), and it is commonly used to delay premature labour by halting uterine contractions. Other oxytocin antagonists include barusiban, nolasiban, epelsiban, and retosiban. Administration of oxytocin antagonists around the time of ET has been proposed as a means to reduce uterine contractions that may interfere with embryo implantation. The intervention involves administering the medication before, during, or after the ET (or a combination).
Objectives: To evaluate the effectiveness and safety of oxytocin antagonists around the time of ET in women undergoing assisted reproduction.
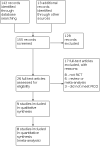
Search methods: We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and two trials registers in March 2021; and checked references and contacted study authors and experts in the field to identify additional studies.
Selection criteria: We included randomised controlled trials (RCTs) of the use of oxytocin antagonists for women undergoing ET, compared with the non-use of this intervention, the use of placebo, or the use of another similar drug.
Data collection and analysis: We used standard methodological procedures recommended by Cochrane. Primary review outcomes were live birth and miscarriage; secondary outcomes were clinical pregnancy and other adverse events.
Main results: We included nine studies (including one comprising three separate trials, 3733 women analysed in total) investigating the role of three different oxytocin antagonists administered intravenously (atosiban), subcutaneously (barusiban), or orally (nolasiban). We found very low- to high-certainty evidence: the main limitations were serious risk of bias due to poor reporting of study methods, and serious or very serious imprecision. Intravenous atosiban versus normal saline or no intervention We are uncertain of the effect of intravenous atosiban on live birth rate (risk ratio (RR) 1.05, 95% confidence interval (CI) 0.88 to 1.24; 1 RCT, N = 800; low-certainty evidence). In a clinic with a live birth rate of 38% per cycle, the use of intravenous atosiban would be associated with a live birth rate ranging from 33.4% to 47.1%. We are uncertain whether intravenous atosiban influences miscarriage rate (RR 1.08, 95% CI 0.75 to 1.56; 5 RCTs, N = 1424; I² = 0%; very low-certainty evidence). In a clinic with a miscarriage rate of 7.2% per cycle, the use of intravenous atosiban would be associated with a miscarriage rate ranging from 5.4% to 11.2%. Intravenous atosiban may increase clinical pregnancy rate (RR 1.50, 95% CI 1.18 to 1.89; 7 RCTs, N = 1646; I² = 69%; low-certainty evidence), and we are uncertain whether multiple or ectopic pregnancy and other complication rates were influenced by the use of intravenous atosiban (very low-certainty evidence). Subcutaneous barusiban versus placebo One study investigated barusiban, but did not report on live birth or miscarriage. We are uncertain whether subcutaneous barusiban influences clinical pregnancy rate (RR 0.96, 95% CI 0.69 to 1.35; 1 RCT, N = 255; very low-certainty evidence). Trialists reported more mild to moderate injection site reactions with barusiban than with placebo, but there was no difference in severe reactions. They reported no serious drug reactions; and comparable neonatal outcome between groups. Oral nolasiban versus placebo Nolasiban does not increase live birth rate (RR 1.13, 95% CI 0.99 to 1.28; 3 RCTs, N = 1832; I² = 0%; high-certainty evidence). In a clinic with a live birth rate of 33% per cycle, the use of oral nolasiban would be associated with a live birth rate ranging from 32.7% to 42.2%. We are uncertain of the effect of oral nolasiban on miscarriage rate (RR 1.45, 95% CI 0.73 to 2.88; 3 RCTs, N = 1832; I² = 0%; low-certainty evidence). In a clinic with a miscarriage rate of 1.5% per cycle, the use of oral nolasiban would be associated with a miscarriage rate ranging from 1.1% to 4.3%. Oral nolasiban improves clinical pregnancy rate (RR 1.15, 95% CI 1.02 to 1.30; 3 RCTs, N = 1832; I² = 0%; high-certainty evidence), and probably does not increase multiple or ectopic pregnancy, or other complication rates (moderate-certainty evidence).
Authors' conclusions: We are uncertain whether intravenous atosiban improves pregnancy outcomes for women undergoing assisted reproductive technology. This conclusion is based on currently available data from seven RCTs, which provided very low- to low-certainty evidence across studies. We could draw no clear conclusions about subcutaneous barusiban, based on limited data from one RCT. Further large well-designed RCTs reporting on live births and adverse clinical outcomes are still required to clarify the exact role of atosiban and barusiban before ET. Oral nolasiban appears to improve clinical pregnancy rate but not live birth rate, with an uncertain effect on miscarriage and adverse events. This conclusion is based on a phased study comprising three trials that provided low- to high-certainty evidence. Further large, well-designed RCTs, reporting on live births and adverse clinical outcomes, should focus on identifying the subgroups of women who are likely to benefit from this intervention.
Trial registration: ClinicalTrials.gov NCT01673399 NCT02893722 NCT03904745.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LC has no conflicts of interest to disclose. NT has no conflicts of interest to disclose. MK has no conflicts of interest to disclose. NRF has received travel costs and honoraria from GE Healthcare, Merck Serono, and Ferring for attending advisory board meetings. Ferring are manufacturers of the oxytocin antagonists atosiban and barusiban. NRF holds shares in two private fertility clinics. MC has received assistance with travel costs from Ferring, Cook Medical, and B. Braun. Ferring are manufacturers of the oxytocin antagonists atosiban and barusiban.
Figures











Update of
References
References to studies included in this review
Ahn 2009 {published data only}
-
- Ahn JW, Kim CH, Kim SR, Jeon GH, Kim SH, Chae HD, et al. Effects of administration of oxytocin antagonist on implantation and pregnancy rates in patients with repeated failure of IVF/ICSI treatment. Korean Journal of Reproductive Medicine 2009;36(4):275-81.
-
- Kim CH, Lee JW, Jeon IK, Park E, Lee YJ, Kim SH, et al. Administration of oxytocin antagonist improves the implantation rates in patients with repeated failure of IVF/ICSI treatment. Human Reproduction (Oxford, England) 2008;23(Suppl 1):i124. [DOI: 10.1093/humrep/den1077] - DOI
Bosch 2019 {published data only}
-
- Bosch E, Aasted H, Klein BM, Arce JC. A randomized, double-blind, placebo-controlled, multi-center, phase 2 trial to investigate the effect of barusiban on implantation in IVF/ICSI patients. Fertility and Sterility 2019;112(3 Suppl 1):e179. [DOI: 10.1016/j.fertnstert.2019.07.584] - DOI
Griesinger 2021 {published data only}
-
- Griesinger G, Blockeel C, Pierzynski P, Tournaye H, Višňová H, Humberstone A, et al. Effect of the oxytocin receptor antagonist nolasiban on pregnancy rates in women undergoing embryo transfer following IVF: analysis of three randomised clinical trials. Human Reproduction (Oxford, England) 2021;36(4):1007-20. [DOI: 10.1093/humrep/deaa369] [PMID: ] - DOI - PubMed
-
- Humberstone A, Terrill P, Macgregor L, Loumaye E. Babies born following administration of nolasiban before embryo transfer (ET) after IVF: neonatal and infant development outcomes from a double-blind, placebo-controlled, clinical trial. Fertility and Sterility 2019;112(3 Suppl 1):e7-8. [DOI: 10.1016/j.fertnstert.2019.07.155] - DOI
-
- Tournaye H, Humberstone A, Lecomte V, Terrill P, Pierzynski P, Loumaye E. A placebo-controlled, randomized, double-blind study of pregnancy and live birth rates after single oral administration of a novel oxytocin antagonist, nolasiban, prior to embryo transfer. Human Reproduction (Oxford, England) 2017;32(Suppl 1):i12. [DOI: 10.1093/humrep/32.Supplement_1.1] - DOI
-
- Visnova H, Tournayeb HJ, Humberstonec A, Terrilld P, Macgregorc L, Loumayec E. A placebo-controlled, randomized, double-blind, phase 3 study assessing ongoing pregnancy rates after single oral administration of a novel oxytocin receptor antagonist, nolasiban, prior to single embryo transfer. Fertility and Sterility 2018;110(4 Supp 1):e45. [DOI: 10.1016/j.fertnstert.2018.07.141] - DOI
He 2016a {published data only}
Hebisha 2018 {published data only}
-
- Hebisha SA, Aboelazm BA, Adel HM, Ahmed AI. Impact of the oxytocin receptor antagonist (atosiban) administered shortly before embryo transfer on pregnancy outcome after intracytoplasmic sperm injection (ICSI). Fertility and Sterility 2016;106(3 Suppl 1):e88. [DOI: 10.1016/j.fertnstert.2016.07.260] - DOI
-
- Hebisha SA, Adel HM. Impact of the oxytocin receptor antagonist atosiban administered shortly before embryo transfer on pregnancy rates after ICSI. Clinical and Experimental Obstetrics and Gynecology 2018;45(4):513-6. [DOI: 10.12891/ceog3907.2018] - DOI
Moraloglu 2010 {published data only}
-
- Moraloglu O, Tonguc E, Batioglu S. Treatment with oxytocin antagonists before embryo transfer may increases the implantation rate after in-vitro fertilization. Human Reproduction (Oxford, England) 2009;24(Suppl 1):i123. [DOI: 10.1093/humrep/dep774] - DOI
Ng 2014 {published data only}
-
- Ng EHY, Li RHW, Chen L, Lan VTN, Tuong HM, Quan S. A randomized double blind comparison of atosiban in unselected patients undergoing in vitro fertilization treatment. Fertility and Sterility 2014;102(3 Suppl 1):e112. [DOI: 10.1016/j.fertnstert.2014.07.386] - DOI
Song 2013 {published data only}
-
- Song XR, Zhao XH, Bai XH, Lu YH, Zhang HJ, Wang YX, et al. Application of oxytocin antagonists in thaw embryo transfer. Chinese Journal of Obstetrics & Gynecology 2013;48(9):667-70. - PubMed
References to studies excluded from this review
Angik 2018 {published data only}
-
- Angik R, Chimote A, Chimote N, Chimote N. Atosiban infusion (oxytocin receptor antagonist) is effective in improving the pregnancy outcome of blastocyst transfer cycle in a younger population (< 35 yrs). Human Reproduction 2018;33(Suppl 1):i325. [DOI: 10.1093/humrep/33.Supplement_1.1] - DOI
Chou 2011 {published data only}
He 2016b {published data only}
-
- He Y, Wu H, He X, Xing Q, Zhou P, Cao Y, et al. Application of atosiban in frozen-thawed cycle patients with different times of embryo transfers. Gynecological Endocrinology 2016;32:811-5. - PubMed
Huang 2017 {published data only}
Kalmantis 2012 {published data only}
-
- Kalmantis K, Loutradis D, Lymperopoulos E, Beretsos P, Bletsa R, Antsaklis A. Three dimensional power doppler evaluation of human endometrium after administration of oxytocine receptor antagonist (OTRa) in an IVF program. Archives of Gynecology and Obstetrics 2012;285(1):265-70. [DOI: 10.1007/s00404-011-2019-2] - DOI - PubMed
Kim 2014 {published data only}
-
- Kim MS, Kim SK, Lee JR, Jee BC, Suh CS, Choi H, et al. The effect of embryo transfer day oxytocin antagonist infusion on IVF outcomes: a systematic review and meta-analysis. Fertility and Sterility 2014;102(3 Suppl 1):e319–20. [DOI: 10.1016/j.fertnstert.2014.07.1082] - DOI
Kim 2016 {published data only}
-
- Kim SK, Han EJ, Kim SM, Lee JR, Jee BC, Suh CS, et al. Efficacy of oxytocin antagonist infusion in improving in vitro fertilization outcomes on the day of embryo transfer: a meta-analysis. Clinical and Experimental Reproductive Medicine 2016;43(4):233-9. [DOI: 10.5653/cerm.2016.43.4.233] - DOI - PMC - PubMed
Lan 2012 {published data only}
Li 2017 {published data only}
Mishra 2018 {published data only}
-
- Mishra V, Agarwal H, Goel S, Roy P, Choudhary S, Lamba S. A prospective case-control trial to evaluate and compare the efficacy and safety of atosiban versus placebo in In vitro fertilization-embryo transfer program. Journal of Human Reproductive Sciences 2018;11(2):155-60. [DOI: 10.4103/jhrs.JHRS_7_17] - DOI - PMC - PubMed
Pierzynski 2007 {published data only}
Pierzynski 2011 {published data only}
Pohl 2020 {published data only}
-
- Pohl O, Marchand L, Pierzynski P, Blockeel C, Mackens S, Lorch U, et al. The mechanism of action of oxytocin receptor antagonists (OTRan) in ART – a study of nolasiban on biomarkers of uterine receptivity in healthy female volunteers. Human Reproduction (Oxford, England) 2020;35(Suppl 1):i83-4. [DOI: 10.1093/humrep/35.Supplement_1.1] - DOI
Schwarze 2020 {published data only}
Visnova 2009 {published data only}
-
- Visnova H, Coroleu B, Piro M, Blockeel C, Mrazek M, Arce JC. Barusiban and atosiban for reduction of uterine contractions on day of embryo transfer do not alter the endocrine profile at time of implantation. Human Reproduction (Oxford, England) 2009;7(24 Supp 1):i180. [DOI: 10.1093/humrep/dep793] - DOI
Visnova 2012 {published data only}
-
- Visnova H, Pierson RA, Mrazek M, Garcia-Velasco JA, Blockeel C, Arce JC. Effects of barusiban, a selective oxytocin antagonist, on uterine contractility in the luteal phase after controlled ovarian stimulation. Fertility and Sterility 2012;98(3 Suppl 1):S183. [DOI: 10.1016/j.fertnstert.2012.07.672] - DOI
Zhang 2014 {published data only}
-
- Zhang Y, Chu Y, Luo H, Zhang Y, Xue F. Influence of atoxiban on pregnancy outcome in patients with repeated implantation failure in freezing embryo transfer cycle. Chinese Journal of Family Planning 2014;22(5):325–8. [http://caod.oriprobe.com/articles/41802527/Influence_of_atoxiban_on_preg...]
References to ongoing studies
ChiCTR1900020795 {published data only}
-
- ChiCTR1900020795. The influence of atosiban on the pregnancy outcome of in vitro fertilization-embryo transfer. www.chictr.org.cn/showproj.aspx?proj=34745 (first registered 19 January 2019).
ChiCTR‐OOR‐16008505 {published data only}
-
- ChiCTR-OOR-16008505. A prospective, randomized, controlled clinical study: could atosiban improve clinical pregnancy rates in patients with two implantation failure in fresh IVF cycles? www.chictr.org.cn/hvshowproject.aspx?id=7790 (record created 20 May 2016).
NCT01673399 {published data only}
-
- NCT01673399. Oxytocin antagonist in patients with repeated failure of implantation. A prospective randomized placebo-controlled double-blind study. clinicaltrials.gov/ct2/show/NCT01673399 (first received 23 August 2012).
NCT02893722 {published data only}
-
- NCT02893722. A randomized double blind comparison of atosiban in patients with RIF undergoing IVF treatment [A randomized double blind comparison of atosiban in patients with repeated implantation failure undergoing IVF treatment]. https://clinicaltrials.gov/ct2/show/NCT02893722 (first posted 8 September 2016).
NCT03904745 {published data only}
-
- NCT03904745. Effect of oxytocin antagonists on implantation success rates of frozen-thawed embryo transfer [Effect of oxytocin antagonists on implantation success rates of frozen-thawed embryo transfer]. https://clinicaltrials.gov/ct2/show/NCT03904745 (first posted 5 April 2019).
Additional references
Akhtar 2013
Ayoubi 2003
-
- Ayoubi JM, Epiney M, Brioschi PA, Fanchin R, Chardonnens D, De Ziegler D. Comparison of changes in uterine contraction frequency after ovulation in the menstrual cycle and in in vitro fertilization cycles. Fertility and Sterility 2003;79(5):1101-5. [DOI: 10.1016/S0015-0282(03)00179-1] [PMID: ] - DOI - PubMed
Craciunas 2014
-
- Craciunas L, Tsampras N, Fitzgerald C. Cervical mucus removal before embryo transfer in women undergoing in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Fertility and Sterility 2014;101(5):1302-7. [DOI: 10.1016/j.fertnstert.2014.01.047] [PMID: ] - DOI - PubMed
Craciunas 2016a
Craciunas 2016b
Craciunas 2018
-
- Craciunas L, Tsampras N, Raine-Fenning N, Coomarasamy A. Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD011537. [DOI: 10.1002/14651858.CD011537.pub3] [PMID: ] - DOI - PMC - PubMed
Craciunas 2019
Decleer 2012
Derks 2009
Fanchin 1998
Fuchs 1985
Glujovsky 2020
-
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD006359. [DOI: 10.1002/14651858.CD006359.pub3] [PMID: ] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 18 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
Knutzen 1992
Kunz 1998
Kunz 2006
Kupka 2014
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lensen 2021
Lesny 1998
RevMan 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Richter 2004
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shukovski 1989
Van der Linden 2015
Vrachnis 2011
Zegers‐Hochschild 2017
Zhu 2012
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

