The mitochondrial permeability transition pore activates the mitochondrial unfolded protein response and promotes aging
- PMID: 34467850
- PMCID: PMC8410078
- DOI: 10.7554/eLife.63453
The mitochondrial permeability transition pore activates the mitochondrial unfolded protein response and promotes aging
Abstract
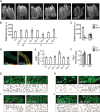
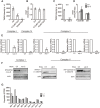
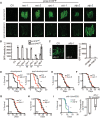
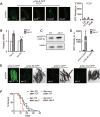
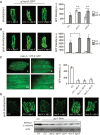
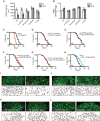
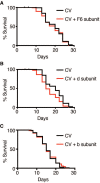
Mitochondrial activity determines aging rate and the onset of chronic diseases. The mitochondrial permeability transition pore (mPTP) is a pathological pore in the inner mitochondrial membrane thought to be composed of the F-ATP synthase (complex V). OSCP, a subunit of F-ATP synthase, helps protect against mPTP formation. How the destabilization of OSCP may contribute to aging, however, is unclear. We have found that loss OSCP in the nematode Caenorhabditis elegans initiates the mPTP and shortens lifespan specifically during adulthood, in part via initiation of the mitochondrial unfolded protein response (UPRmt). Pharmacological or genetic inhibition of the mPTP inhibits the UPRmt and restores normal lifespan. Loss of the putative pore-forming component of F-ATP synthase extends adult lifespan, suggesting that the mPTP normally promotes aging. Our findings reveal how an mPTP/UPRmt nexus may contribute to aging and age-related diseases and how inhibition of the UPRmt may be protective under certain conditions.
Keywords: C. elegans; F-ATP synthase; aging; c-subunit; cell biology; mitochondrial permeability transition pore; mitochondrial unfolded protein response; oscp/atp-3.
© 2021, Angeli et al.
Conflict of interest statement
SA, AF, MC, TP, AG, AS, JA, GL No competing interests declared
Figures

References
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. PNAS. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. - DOI - PMC - PubMed
-
- Amodeo GF, Lee BY, Krilyuk N, Filice CT, Valyuk D, Otzen DE, Noskov S, Leonenko Z, Pavlov EV. C subunit of the ATP synthase is an amyloidogenic calcium dependent channel-forming peptide with possible implications in mitochondrial permeability transition. Scientific Reports. 2021;11:8744. doi: 10.1038/s41598-021-88157-z. - DOI - PMC - PubMed
-
- Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V, Giorgio V, Carraro M, Di Lisa F, Forte M, Szabó I, Lippe G, Bernardi P. The unique histidine in OSCP subunit of F-ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Reports. 2018;19:257–268. doi: 10.15252/embr.201744705. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

