Ibrexafungerp Versus Placebo for Vulvovaginal Candidiasis Treatment: A Phase 3, Randomized, Controlled Superiority Trial (VANISH 303)
- PMID: 34467969
- PMCID: PMC9187327
- DOI: 10.1093/cid/ciab750
Ibrexafungerp Versus Placebo for Vulvovaginal Candidiasis Treatment: A Phase 3, Randomized, Controlled Superiority Trial (VANISH 303)
Abstract
Background: Current treatment of vulvovaginal candidiasis (VVC) is largely limited to azole therapy. Ibrexafungerp is a first-in-class triterpenoid antifungal with broad-spectrum anti-Candida fungicidal activity. The objective of this study was to evaluate the efficacy and safety of ibrexafungerp compared with placebo in patients with acute VVC.
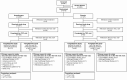
Methods: Patients were randomly assigned 2:1 to receive ibrexafungerp (300 mg twice for 1 day) or placebo. The primary endpoint was the percentage of patients with a clinical cure (complete resolution of vulvovaginal signs and symptoms [VSS] = 0) at test-of-cure (day 11 ± 3). Secondary endpoints included the percentage of patients with mycological eradication, overall success (clinical cure and mycological eradication), clinical improvement (VSS ≤ 1) at test-of-cure, and symptom resolution at follow-up (day 25 ± 4).
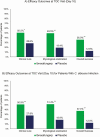
Results: Patients receiving ibrexafungerp had significantly higher rates of clinical cure (50.5% [95/188] vs 28.6% [28/98]; P = .001), mycological eradication (49.5% [93/188] vs 19.4% [19/98]; P < .001), and overall success (36.0% [64/178] vs 12.6% [12/95]; P < .001) compared with placebo. Symptom resolution was sustained and further increased with ibrexafungerp compared with placebo (59.6% [112/188] vs 44.9% [44/98]; P = .009) at follow-up. Post hoc analysis showed similar rates of clinical cure and clinical improvement at test-of-cure for Black patients (54.8% [40/73] and 63.4% [47/73], respectively) and patients with a body mass index >35 (54.5% [24/44] and 68.2% [30/44], respectively) compared with overall rates. Ibrexafungerp was well tolerated. Adverse events were primarily gastrointestinal and mild in severity.
Conclusions: Ibrexafungerp provides a promising safe and efficacious oral treatment that mechanistically differs from current azole treatment options for acute VVC.
Keywords: Candida albicans; SCY-078; ibrexafungerp; placebo; vulvovaginal candidiasis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Ibrexafungerp to treat acute vulvovaginal candidiasis.Nat Rev Urol. 2021 Nov;18(11):638. doi: 10.1038/s41585-021-00522-9. Nat Rev Urol. 2021. PMID: 34548655 No abstract available.
References
-
- Mendling W, Brasch J, Cornely OA, et al. . Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015; 58:1–15. - PubMed
-
- Jeanmonod R, Jeanmonod D. Vaginal candidiasis. Available at: https://www.ncbi.nlm.nih.gov/books/. Accessed 9 March 2021.
-
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016; 214:15–21. - PubMed

