Continuous-Flow Left Ventricular Assist Device Support in Patients with Ischemic Versus Nonischemic Cardiomyopathy
- PMID: 34468765
- PMCID: PMC8717751
- DOI: 10.14503/THIJ-20-7241
Continuous-Flow Left Ventricular Assist Device Support in Patients with Ischemic Versus Nonischemic Cardiomyopathy
Abstract
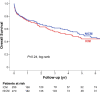
To determine whether the cause of cardiomyopathy affects outcomes in patients who undergo continuous-flow left ventricular assist device support, we compared postimplant adverse events and survival between patients with ischemic and nonischemic cardiomyopathy. The inclusion criteria for the ischemic group were a history of myocardial infarction or revascularization (coronary artery bypass grafting or percutaneous coronary intervention), ≥75% stenosis of the left main or proximal left anterior descending coronary artery, or ≥75% stenosis of ≥2 epicardial vessels. From November 2003 through March 2016, 526 patients underwent device support: 256 (48.7%) in the ischemic group and 270 (51.3%) in the nonischemic group. The ischemic group was older (60.0 vs 50.0 yr), included more men than women (84.0% vs 72.6%), and had more comorbidities. More patients in the nonischemic group were able to have their devices explanted after left ventricular recovery (5.9% vs 2.0%; P=0.02). More patients in the ischemic group had gastrointestinal bleeding (31.2% vs 22.6%; P=0.03), particularly from arteriovenous malformations (20.7% vs 11.9%; P=0.006) and ulcers (16.4% vs 9.3%; P=0.01). Kaplan-Meier analysis revealed no difference in overall survival between groups (P=0.24). Older age, previous sternotomy, higher total bilirubin level, and concomitant procedures during device implantation independently predicted death (P ≤0.03), whereas cause of heart failure did not (P=0.08). Despite the similarity in overall survival between groups, ischemic cardiomyopathy was associated with more frequent gastrointestinal bleeding. This information may help guide the care of patients with ischemic cardiomyopathy who receive continuous-flow left ventricular assist device support.
Keywords: Cardiomyopathy; heart failure/etiology; heart-assist devices/adverse effects; survival; treatment outcome.
© 2021 by the Texas Heart® Institute, Houston.
Conflict of interest statement
Figures
References
-
- Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant . 2017;36(10):1080–6. - PubMed
-
- Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials [published erratum appears in JAMA 1995;274(6):462] JAMA. 1995;273(18):1450–6. - PubMed
-
- Likoff MJ, Chandler SL, Kay HR. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol . 1987;59(6):634–8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical