Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial
- PMID: 34468869
- PMCID: PMC8732941
- DOI: 10.1007/s10120-021-01227-z
Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial
Abstract
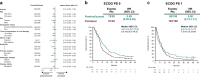
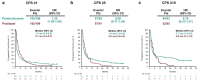
Background: In the phase 3 KEYNOTE-061 study (cutoff: 10/26/2017), pembrolizumab did not significantly prolong OS vs paclitaxel as second-line (2L) therapy in PD-L1 combined positive score (CPS) ≥ 1 gastric/GEJ cancer. We present results in CPS ≥ 1, ≥ 5, and ≥ 10 populations after two additional years of follow-up (cutoff: 10/07/2019).
Methods: Patients were randomly allocated 1:1 to pembrolizumab 200 mg Q3W for ≤ 35 cycles or standard-dose paclitaxel. Primary endpoints: OS and PFS (CPS ≥ 1 population). HRs were calculated using stratified Cox proportional hazards models.
Results: 366/395 patients (92.7%) with CPS ≥ 1 died. Pembrolizumab demonstrated a trend toward improved OS vs paclitaxel in the CPS ≥ 1 population (HR, 0.81); 24-month OS rates: 19.9% vs 8.5%. Pembrolizumab incrementally increased the OS benefit with PD-L1 enrichment (CPS ≥ 5: HR, 0.72, 24-month rate, 24.2% vs 8.8%; CPS ≥ 10: 0.69, 24-month rate, 32.1% vs 10.9%). There was no difference in median PFS among treatment groups (CPS ≥ 1: HR, 1.25; CPS ≥ 5: 0.98; CPS ≥ 10: 0.79). ORR (pembrolizumab vs paclitaxel) was 16.3% vs 13.6% (CPS ≥ 1), 20.0% vs 14.3% (CPS ≥ 5), and 24.5% vs 9.1% (CPS ≥ 10); median DOR was 19.1 months vs 5.2, 32.7 vs 4.8, and NR vs 6.9, respectively. Fewer treatment-related AEs (TRAEs) occurred with pembrolizumab than paclitaxel (53% vs 84%).
Conclusion: In this long-term analysis, 2L pembrolizumab did not significantly improve OS but was associated with higher 24-month OS rates than paclitaxel. Pembrolizumab also increased OS benefit with PD-L1 enrichment among patients with PD-L1-positive gastric/GEJ cancer and led to fewer TRAEs than paclitaxel.
Trial registration: ClinicalTrials.gov, NCT02370498.
Keywords: Chemotherapy; Gastric cancer; Gastroesophageal junction cancer; Pembrolizumab.
© 2021. The Author(s).
Conflict of interest statement
Charles S. Fuchs reports board membership (director of board) for CytomX; consultancy for Agios, Amylin Pharmaceuticals, AstraZeneca, Bain Capital, CytomX Therapeutics, Daiichi-Sankyo, Eli Lilly, Entrinsic Health, Genentech, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Taiho, and Unum Therapeutics; expert testimony for Amylin Therapeutics and Eli Lilly; unexercised stock options for CytomX and Entrinsic Health; and co-founder of EvolveImmune Therapeutics. Mustafa Özgüroğlu has nothing to disclose. Yung-Jue Bang reports consultancy for Astellas Pharma, AstraZeneca, Bayer, BeiGene, BMS, Daiichi-Sankyo, Eli Lilly, Genentech/Roche, Genexine, GreenCross, Hanmi, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Novartis, Samyang Biopharm, and Taiho; and grants from Astellas Pharma, AstraZeneca, Bayer, BeiGene, Boehringer-Ingelheim, BMS, Boston Biomedical, CKD Pharma, Curis, Daiichi-Sankyo, Eli Lilly, FivePrime, Genentech/Roche, Genexine, GreenCross, GSK, Macrogenics, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Novartis, Ono, Pfizer, Taiho, and Takeda. Maria Di Bartolomeo has nothing to disclose. Mario Mandala reports board membership for Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche; and grants from BMS, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche. Min-Hee Ryu reports honoraria from and advisory boards for AstraZeneca, BMS, Daehwa Pharmaceutical, Lilly, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Ono, and Taiho; payment for lectures from BMS, Daehwa Pharmaceutical, Lilly, and Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Lorenzo Fornaro reports board membership for Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; payment for lectures, including service on speakers bureaus, from Eli Lilly; and travel/accommodations/meeting expenses from Celgene. Tomasz Olesinski has nothing to disclose. Christian Caglevic reports consultancy for Andes Biotechnologies, Boehringer Ingelheim, BMS, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche; payment for lectures, including service on speakers bureaus, from BMS, Lilly, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche; travel/accommodations/meeting expenses from Roche; and service as a clinical trial investigator for Astellas Pharma, AstraZeneca, BMS, GSK, Medivation, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche. Hyun C. Chung reports consultancy for Amgen, BeiGene, BMS, Celltrion, Gloria, Lilly, Merck-Serono, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Taiho, Zymework; and grants from Amgen, BeiGene, BMS/Ono, GSK Incyte, Lilly, Merck-Serono, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Taiho. Kei Muro reports grants from Astellas Pharma, Amgen Biopharma, Daiichi Sankyo, Merck-Serono, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Parexel International, Ono, Pfizer, Sanofi, Solasia Pharma, and Taiho; and consulting fees or honoraria from Amgen, AstraZeneca, Bayer, BMS, Chugai, Eli Lilly, Ono Pharmaceutical, Sanofi, Taiho, and Takeda. Eric Van Cutsem reports consultancy for Array, Astellas Pharma, AstraZeneca, Bayer, Biocartis, BMS, Celgene, Daichi-Sankyo, GSK, Halozyme, Incyte, Ipsen, Lilly, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck KGaA, Novartis, Pierre-Fabre, Roche, Servier, Sirtex, and Taiho; and grants from Amgen, Bayer, Boehringer-Ingelheim, BSM, Celgene, Ipsen, Lilly, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck KGaA, Novartis, Roche, and Servier. Anneli Elme has nothing to disclose. Peter Thuss-Patience reports consulting fees or honoraria from AstraZeneca, BMS, Lilly, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Pfizer, Roche, and Servier. Ian Chau reports advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, Five Prime Therapeutics, Merck-Serono, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Oncologie International, Pierre Fabre, Roche; research funding from Eli Lilly, Janssen-Cilag, and Sanofi Oncology; and honorarium from Eli Lilly. Atsushi Ohtsu reports personal fees from BMS, Chugai, Ono, and Taiho. Pooja Bhagia is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Anran Wang is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Chie-Schin Shih is an employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Kohei Shitara reports consultancy with AbbVie, Astellas Pharma, BMS, Eli Lilly, GSK, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, and Takeda Pharmaceutical; grants from Astellas Pharma, Chugai Pharma, Dainippon Sumitomo Pharma, Daiichi Sanyko, Eli Lilly, Medi Science, Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Ono Pharmaceutical, and Taiho Pharmaceutical; and honoraria (lecture fees) from AbbVie, Novartis, and Yakult.
Figures


References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): gastric cancer (Version 4.2020). 2020. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.o.... Accessed 8 Feb 2021.
-
- KEYTRUDA® (2021) (pembrolizumab) injection, for intravenous use. 03/2021. Merck Sharp & Dohme Corp.: Whitehouse Station, NJ, USA
-
- Wainberg ZA, Jalal S, Muro K, Yoon HH, Grrido M, Golan T, et al. KEYNOTE-059 update: efficacy and safety of pembrolizumab alone or in combination with chemotherapy in patients with advanced gastric or gastroesophageal (G/GEJ) cancer. Ann Oncol. 2017;28:616. doi: 10.1093/annonc/mdx440.020. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

