Anti-inflammatory activity of palmitoylethanolamide ameliorates osteoarthritis induced by monosodium iodoacetate in Sprague-Dawley rats
- PMID: 34468900
- PMCID: PMC8514352
- DOI: 10.1007/s10787-021-00870-3
Anti-inflammatory activity of palmitoylethanolamide ameliorates osteoarthritis induced by monosodium iodoacetate in Sprague-Dawley rats
Abstract
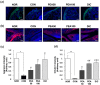
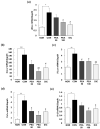
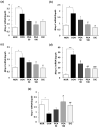
Novel treatment strategies are urgently required for osteoarthritis (OA). Palmitoylethanolamide (PEA) is a naturally occurring fatty acid amide with analgesic and anti-inflammatory effects. We aimed to examine its effect on OA and elucidate the molecular mechanism of actions in monosodium iodoacetate (MIA)-induced OA Sprague-Dawley rats. The experimental animals were divided into normal control group (injected with saline + treated with phosphate-buffered saline (PBS), NOR), control group (injected with MIA + treated with PBS, CON), 50 or 100 mg/kg body weight (BW)/day PEA-treated group (injected with MIA + treated with 50 or 100 mg of PEA/kg BW/day, PEA50 or PEA100), and positive control group (injected with MIA + treated with 6 mg of diclofenac/kg BW/day, DiC). The changes in blood parameters, body parameters, gene expression of inflammatory mediators and cytokines, knee thickness, and joint tissue were observed. Oral administration of PEA had no adverse effects on the BW, liver, or kidneys. PEA reduced knee joint swelling and cartilage degradation in MIA-induced OA rats. The serum levels of leukotriene B4, nitric oxide, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and prostaglandin E2 considerably reduced in the PEA100 group compared with those in the CON group. In the synovia of knee joints, the mRNA expression of iNOS, 5-Lox, Cox-2, Il-1β, Tnf-α, and Mmp-2, -3, -9, and -13 apparently increased with MIA administration. Meanwhile, Timp-1 mRNA expression apparently decreased in the CON group but increased to the normal level with PEA treatment. Thus, PEA can be an effective therapeutic agent for OA.
Keywords: Cytokine; Inflammation; Monosodium iodoacetate (MIA); Osteoarthritis; Palmitoylethanolamide (PEA).
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models.BMC Vet Res. 2017 Aug 2;13(1):229. doi: 10.1186/s12917-017-1151-z. BMC Vet Res. 2017. PMID: 28768536 Free PMC article.
-
Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis.Nutrients. 2020 May 19;12(5):1477. doi: 10.3390/nu12051477. Nutrients. 2020. PMID: 32438772 Free PMC article.
-
Evaluating the Anti-Osteoarthritis Potential of Standardized Boswellia serrata Gum Resin Extract in Alleviating Knee Joint Pathology and Inflammation in Osteoarthritis-Induced Models.Int J Mol Sci. 2024 Mar 12;25(6):3218. doi: 10.3390/ijms25063218. Int J Mol Sci. 2024. PMID: 38542192 Free PMC article.
-
Palmitoylethanolamide and White Matter Lesions: Evidence for Therapeutic Implications.Biomolecules. 2022 Aug 27;12(9):1191. doi: 10.3390/biom12091191. Biomolecules. 2022. PMID: 36139030 Free PMC article. Review.
-
Micronized / ultramicronized palmitoylethanolamide (PEA) as natural neuroprotector against COVID-19 inflammation.Prostaglandins Other Lipid Mediat. 2021 Jun;154:106540. doi: 10.1016/j.prostaglandins.2021.106540. Epub 2021 Feb 23. Prostaglandins Other Lipid Mediat. 2021. PMID: 33636368 Free PMC article. Review.
Cited by
-
Machine learning for precision diagnostics of autoimmunity.Sci Rep. 2024 Nov 13;14(1):27848. doi: 10.1038/s41598-024-76093-7. Sci Rep. 2024. PMID: 39537649 Free PMC article.
-
Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials.Nutrients. 2023 Mar 10;15(6):1350. doi: 10.3390/nu15061350. Nutrients. 2023. PMID: 36986081 Free PMC article.
-
Palmitoylethanolamide, an endogenous fatty acid amide, and its pleiotropic health benefits: A narrative review.J Biomed Res. 2024 Oct 22;39(3):215-228. doi: 10.7555/JBR.38.20240053. J Biomed Res. 2024. PMID: 39433509 Free PMC article.
-
Anti-osteoarthritis effect of a standardized Curcuma longa extract in monosodium iodoacetate-induced osteoarthritis rats.Nutr Res Pract. 2025 Aug;19(4):537-553. doi: 10.4162/nrp.2025.19.4.537. Epub 2025 Mar 10. Nutr Res Pract. 2025. PMID: 40809890 Free PMC article.
-
Evaluation of the anti-osteoarthritic effects and mechanisms of Cissus quadrangularis extract containing quercetin and isorhamnetin in a rat model of monosodium iodoacetate-induced osteoarthritis.Food Nutr Res. 2025 Apr 15;69. doi: 10.29219/fnr.v69.12173. eCollection 2025. Food Nutr Res. 2025. PMID: 40264489 Free PMC article.
References
-
- Alsalem M, Haddad M, Aldossary SA, Kalbouneh H, Altarifi A, Jaffal SM, Abbas MA, Aldaoud N, El-Salem K. Role of cannabinoid receptor 1 and the peroxisome proliferator-activated receptor α in mediating anti-nociceptive effects of synthetic cannabinoids and a cannabinoid-like compound. Inflammopharmacology. 2019;27:1131–1142. doi: 10.1007/s10787-019-00584-7. - DOI - PubMed
-
- Audial S, Bonnotte B. Inflammation. Rev Prat. 2015;65:403–408. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
