Glycan Array Evaluation of Synthetic Epitopes between the Capsular Polysaccharides from Streptococcus pneumoniae 19F and 19A
- PMID: 34469105
- PMCID: PMC8453487
- DOI: 10.1021/acschembio.1c00347
Glycan Array Evaluation of Synthetic Epitopes between the Capsular Polysaccharides from Streptococcus pneumoniae 19F and 19A
Abstract
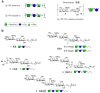
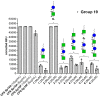
Vaccination represents the most effective way to prevent invasive pneumococcal diseases. The glycoconjugate vaccines licensed so far are obtained from capsular polysaccharides (CPSs) of the most virulent serotypes. Protection is largely limited to the specific vaccine serotypes, and the continuous need for broader coverage to control the outbreak of emerging serotypes is pushing the development of new vaccine candidates. Indeed, the development of efficacious vaccine formulation is complicated by the high number of bacterial serotypes with different CPSs. In this context, to simplify vaccine composition, we propose the design of new saccharide fragments containing chemical structures shared by different serotypes as cross-reactive and potentially cross-protective common antigens. In particular, we focused on Streptococcus pneumoniae (Sp) 19A and 19F. The CPS repeating units of Sp 19F and 19A are very similar and share a common structure, the disaccharide ManNAc-β-(1→4)-Glc (A-B). Herein, we describe the synthesis of a small library of compounds containing different combinations of the common 19F/19A disaccharide. The six new compounds were tested with a glycan array to evaluate their recognition by antibodies in reference group 19 antisera and factor reference antisera (reacting against 19F or 19A). The disaccharide A-B, phosphorylated at the upstream end, emerged as a hit from the glycan array screening because it is strongly recognized by the group 19 antisera and by the 19F and 19A factor antisera, with similar intensity compared with the CPSs used as controls. Our data give a strong indication that the phosphorylated disaccharide A-B can be considered a common epitope among different Sp 19 serotypes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Characterization of the cross-reaction between type 19F(19) and 19A(57) pneumococcal capsular polysaccharides: compositional analysis and immunological relation determined with rabbit typing antisera.Infect Immun. 1978 Dec;22(3):727-35. doi: 10.1128/iai.22.3.727-735.1978. Infect Immun. 1978. PMID: 83302 Free PMC article.
-
Synthesis and biological evaluation of a trisaccharide repeating unit derivative of Streptococcus pneumoniae 19A capsular polysaccharide.Bioorg Med Chem. 2018 Nov 15;26(21):5682-5690. doi: 10.1016/j.bmc.2018.10.016. Epub 2018 Oct 19. Bioorg Med Chem. 2018. PMID: 30449426
-
Chimeric oligosaccharide conjugate induces opsonic antibodies against Streptococcus pneumoniae serotypes 19A and 19F.Chem Sci. 2020 Jun 26;11(28):7401-7407. doi: 10.1039/d0sc02230f. Chem Sci. 2020. PMID: 34123020 Free PMC article.
-
Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?BMC Pediatr. 2010 Feb 2;10:4. doi: 10.1186/1471-2431-10-4. BMC Pediatr. 2010. PMID: 20122261 Free PMC article. Review.
-
Bacterial capsular polysaccharides--biochemistry, immunity and vaccine.Mol Immunol. 1987 Oct;24(10):1005-19. doi: 10.1016/0161-5890(87)90067-8. Mol Immunol. 1987. PMID: 3119993 Review.
Cited by
-
Strengths and weaknesses of pneumococcal conjugate vaccines.Glycoconj J. 2023 Apr;40(2):135-148. doi: 10.1007/s10719-023-10100-3. Epub 2023 Jan 18. Glycoconj J. 2023. PMID: 36652051 Free PMC article. Review.
-
Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance.Chem Rev. 2022 Oct 26;122(20):15672-15716. doi: 10.1021/acs.chemrev.2c00021. Epub 2022 May 24. Chem Rev. 2022. PMID: 35608633 Free PMC article. Review.
-
Serum antibody screening using glycan arrays.Chem Soc Rev. 2024 Mar 4;53(5):2603-2642. doi: 10.1039/d3cs00693j. Chem Soc Rev. 2024. PMID: 38305761 Free PMC article. Review.
References
-
- Klugman K. P.; Black S.; Dagan R.; Malley R.; Whitney C. G.. 25 - Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In Vaccines, 6th ed.; Plotkin S. A., Orenstein W. A., Offit P. A., Eds.; W. B. Saunders: London, 2013; pp 504–541.
-
- Klugman K. P.; Dagan R.; Malley R.; Whitney C. G.. 46 - Pneumococcal Conjugate Vaccine and Pneumococcal Common Protein Vaccines, In Plotkin’s Vaccines, 7th ed.; Plotkin S. A., Orenstein W. A., Offit P. A., Edwards K. M., Eds.; Elsevier, 2018; pp 773–815.e718.
-
- experts_pneumococcal_vaccines. https://www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

