Pulmonary Innate Lymphoid Cell Responses during Rhinovirus-induced Asthma Exacerbations In Vivo: A Clinical Trial
- PMID: 34469272
- PMCID: PMC8786078
- DOI: 10.1164/rccm.202010-3754OC
Pulmonary Innate Lymphoid Cell Responses during Rhinovirus-induced Asthma Exacerbations In Vivo: A Clinical Trial
Abstract
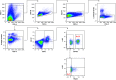
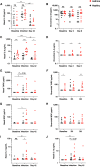
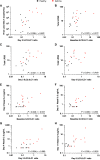
Rationale: Type 2 innate lymphoid cells (ILC2s) are significant sources of type 2 cytokines, which are implicated in the pathogenesis of asthma and asthma exacerbations. The role of ILC2s in virus-induced asthma exacerbations is not well characterized. Objectives: To characterize pulmonary ILC responses following experimental rhinovirus challenge in patients with moderate asthma and healthy subjects. Methods: Patients with moderate asthma and healthy subjects were inoculated with rhinovirus-16 and underwent bronchoscopy at baseline and at Day 3, and Day 8 after inoculation. Pulmonary ILC1s and ILC2s were quantified in bronchoalveolar lavage using flow cytometry. The ratio of bronchoalveolar lavage ILC2:ILC1 was assessed to determine their relative contributions to the clinical and immune response to rhinovirus challenge. Measurements and Main Results: At baseline, ILC2s were significantly higher in patients with asthma than in healthy subjects. At Day 8, ILC2s significantly increased from baseline in both groups, which was significantly higher in patients with asthma than in healthy subjects (all comparisons P < 0.05). In healthy subjects, ILC1s increased from baseline at Day 3 (P = 0.001), while in patients with asthma, ILC1s increased from baseline at Day 8 (P = 0.042). Patients with asthma had significantly higher ILC2:ILC1 ratios at baseline (P = 0.024) and Day 8 (P = 0.005). Increased ILC2:ILC1 ratio in patients with asthma correlated with clinical exacerbation severity and type 2 cytokines in nasal mucosal lining fluid. Conclusions: An ILC2-predominant inflammatory profile in patients with asthma was associated with increased severity and duration of rhinovirus infection compared with healthy subjects, supporting the potential role of ILC2s in the pathogenesis of virus-induced asthma exacerbations.
Keywords: ILC1; ILC2; asthma; innate lymphoid cells; rhinovirus.
Figures




Comment in
-
ILC2s in Virus-induced Asthma Exacerbations: A Starring or Supportive Role?Am J Respir Crit Care Med. 2021 Dec 1;204(11):1239-1240. doi: 10.1164/rccm.202108-2001ED. Am J Respir Crit Care Med. 2021. PMID: 34582713 Free PMC article. No abstract available.
-
Weaving innate lymphoid cells (ILCs) into the fabric of asthma exacerbations.J Allergy Clin Immunol. 2022 May;149(5):1579-1581. doi: 10.1016/j.jaci.2022.01.021. Epub 2022 Feb 8. J Allergy Clin Immunol. 2022. PMID: 35149042 No abstract available.
References
-
- Jackson DJ, Trujillo-Torralbo MB, del-Rosario J, Bartlett NW, Edwards MR, Mallia P, et al. The influence of asthma control on the severity of virus-induced asthma exacerbations. J Allergy Clin Immunol . 2015;136:497–500.e3. - PubMed
-
- Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med . 2009;360:985–993. - PubMed

