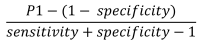Probability-Based Estimates of Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence and Detection Fraction, Utah, USA
- PMID: 34469285
- PMCID: PMC8544980
- DOI: 10.3201/eid2711.204435
Probability-Based Estimates of Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence and Detection Fraction, Utah, USA
Abstract
We aimed to generate an unbiased estimate of the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in 4 urban counties in Utah, USA. We used a multistage sampling design to randomly select community-representative participants >12 years of age. During May 4-June 30, 2020, we collected serum samples and survey responses from 8,108 persons belonging to 5,125 households. We used a qualitative chemiluminescent microparticle immunoassay to detect SARS-CoV-2 IgG in serum samples. We estimated the overall seroprevalence to be 0.8%. The estimated seroprevalence-to-case count ratio was 2.5, corresponding to a detection fraction of 40%. Only 0.2% of participants from whom we collected nasopharyngeal swab samples had SARS-CoV-2-positive reverse transcription PCR results. SARS-CoV-2 antibody prevalence during the study was low, and prevalence of PCR-positive cases was even lower. The comparatively high SARS-CoV-2 detection rate (40%) demonstrates the effectiveness of Utah's testing strategy and public health response.
Keywords: COVID-19; IgG; PCR; SARS; SARS-CoV-2; United States; Utah; antibodies; case detection; coronavirus; coronavirus disease; immunoglobulin G; incidence; infections; nasopharyngeal swabs; population surveillance; probability sampling design; rRT-PCR; respiratory infections; reverse transcription PCR; sensitivity; seroepidemiologic studies; serology; seroprevalence; severe acute respiratory syndrome coronavirus 2; specificity; viruses; zoonoses.
Figures
References
-
- Center for Systems Science and Engineering at Johns Hopkins University. COVID-19 dashboard. 2020. [cited 2021 May 3]. https://coronavirus.jhu.edu/map.html
-
- United States Census Bureau. Quick facts 2020. [cited 2020 October 23]. https://www.census.gov/quickfacts/fact/table/US,daviscountyutah,summitco...
-
- Kem C. Gardner Policy Institute of the University of Utah. US Census Bureau estimates for race and Hispanic origin, vintage 2019. [cited 2020 Oct 5]. https://gardner.utah.edu/demographics/current-census-bureau-estimates
-
- Abbott Laboratories Diagnostics Division. SARS-CoV-2 IgG reagent kit for use with Architect, June 2020. [package insert] [cited 2021 May 3]. https://www.fda.gov/media/137383/download
-
- Abbott Laboratories Diagnostics Division. SARS-CoV-2 IgG calibrator kit for use with Architect, April 2020. [package insert] [cited 2021 May 3]. https://www.henryschein.com/assets/Medical/Abbott%20IFU%20-%20Calibratio...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous