Effectiveness of Abbott BinaxNOW Rapid Antigen Test for Detection of SARS-CoV-2 Infections in Outbreak among Horse Racetrack Workers, California, USA
- PMID: 34469287
- PMCID: PMC8544964
- DOI: 10.3201/eid2711.211449
Effectiveness of Abbott BinaxNOW Rapid Antigen Test for Detection of SARS-CoV-2 Infections in Outbreak among Horse Racetrack Workers, California, USA
Abstract
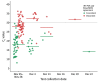
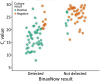
The Abbott BinaxNOW rapid antigen test is cheaper and faster than real-time reverse transcription PCR (rRT-PCR) for detecting severe acute respiratory syndrome coronavirus 2. We compared BinaxNOW with rRT-PCR in 769 paired specimens from 342 persons during a coronavirus disease outbreak among horse racetrack workers in California, USA. We found positive percent agreement was 43.3% (95% CI 34.6%-52.4%), negative percent agreement 100% (95% CI 99.4%-100%), positive predictive value 100% (95% CI 93.5%-100%), and negative predictive value 89.9% (95% CI 87.5%-92.0%). Among 127 rRT-PCR-positive specimens, the 55 with paired BinaxNOW-positive results had a lower mean cycle threshold than the 72 with paired BinaxNOW-negative results (17.8 vs. 28.5; p<0.001). Of 100 specimens with cycle threshold <30, a total of 51 resulted in positive virus isolation; 45 (88.2%) of those were BinaxNOW-positive. Our comparison supports immediate isolation for BinaxNOW-positive persons and confirmatory testing for negative persons.
Keywords: COVID-19; SARS-CoV-2; SARS-CoV-2 RT-PCR testing; SARS-CoV-2 antigen testing; coronavirus disease; disease outbreaks; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; workplace; zoonoses.
Figures
References
-
- Food and Drug Administration. BinaxNOW COVID-19 Ag card (PN 195–000)—instructions for use. 2020. Dec [cited 2021 Mar 15]. https://www.fda.gov/media/141570/download
-
- Centers for Disease Control and Prevention. Interim guidance for antigen testing for SARS-CoV-2. 2020. Dec 5 [cited 2021 Mar 15]. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu...
-
- James AE, Gulley T, Kothari A, Holder K, Garner K, Patil N. Performance of the BinaxNOW COVID-19 antigen card test relative to the SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction assay among symptomatic and asymptomatic healthcare employees. Infect Control Hosp Epidemiol. 2021; [Epub ahead of print]. - PMC - PubMed
-
- Pilarowski G, Lebel P, Sunshine S, Liu J, Crawford E, Marquez C, et al. Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis. 2021;223:1139–44. 10.1093/infdis/jiaa802 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

