Estimating Vaccine Efficacy Against Transmission via Effect on Viral Load
- PMID: 34469363
- PMCID: PMC8478108
- DOI: 10.1097/EDE.0000000000001415
Estimating Vaccine Efficacy Against Transmission via Effect on Viral Load
Abstract
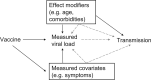
Determining policies to end the SARS-CoV-2 pandemic will require an understanding of the efficacy and effectiveness (hereafter, efficacy) of vaccines. Beyond the efficacy against severe disease and symptomatic and asymptomatic infection, understanding vaccine efficacy against virus transmission, including efficacy against transmission of different viral variants, will help model epidemic trajectory and determine appropriate control measures. Recent studies have proposed using random virologic testing in individual randomized controlled trials to improve estimation of vaccine efficacy against infection. We propose to further use the viral load measures from these tests to estimate efficacy against transmission. This estimation requires a model of the relationship between viral load and transmissibility and assumptions about the vaccine effect on transmission and the progress of the epidemic. We describe these key assumptions, potential violations of them, and solutions that can be implemented to mitigate these violations. Assessing these assumptions and implementing this random sampling, with viral load measures, will enable better estimation of the crucial measure of vaccine efficacy against transmission.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
L.K.-S. reports no competing interests. M.L. reports consulting/honoraria from Bristol Myers Squibb, Sanofi Pasteur, Janssen, and Merck, as well as a grant through his institution, unrelated to COVID-19, from Pfizer. He has served as an unpaid advisor related to COVID-19 to Pfizer, One Day Sooner, Astra-Zeneca, Janssen, and COVAX (United Biomedical). R.K. discloses consulting fees from Partners in Health.
Figures

References
-
- Lipsitch M, Dean NE. Understanding COVID-19 vaccine efficacy. Science. 2020;370:763–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

