Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL
- PMID: 34469514
- PMCID: PMC11017791
- DOI: 10.1182/blood.2021012775
Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL
Abstract
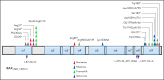
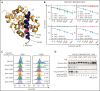
The BCL2 inhibitor venetoclax has established therapeutic roles in chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). As BCL2 is an important determinant of survival of both myeloid progenitor and B cells, we investigated whether clinical and molecular abnormalities arise in the myeloid compartment during long-term continuous venetoclax treatment of CLL in 89 patients (87 with relapsed/refractory CLL). Over a median follow-up of 75 (range 21-98) months, persistent cytopenias (≥1 of neutropenia, thrombocytopenia, anemia) lasting ≥4 months and unrelated to CLL occurred in 25 patients (28%). Of these patients, 20 (80%) displayed clonal hematopoiesis, including 10 with therapy-related myeloid neoplasms (t-MNs). t-MNs occurred exclusively in patients previously exposed to fludarabine-alkylator combination therapy with a cumulative 5-year incidence of 10.4% after venetoclax initiation, consistent with rates reported for patients exposed to fludarabine-alkylator combination therapy without venetoclax. To determine whether the altered myelopoiesis reflected the acquisition of mutations, we analyzed samples from patients with no or minimal bone marrow CLL burden (n = 41). Mutations in the apoptosis effector BAX were identified in 32% (13/41). In cellular assays, C-terminal BAX mutants abrogated outer mitochondrial membrane localization of BAX and engendered resistance to venetoclax killing. BAX-mutated clonal hematopoiesis occurred independently of prior fludarabine-alkylator combination therapy exposure and was not associated with t-MNs. Single-cell sequencing revealed clonal co-occurrence of mutations in BAX with DNMT3A or ASXL1. We also observed simultaneous BCL2 mutations within CLL cells and BAX mutations in the myeloid compartment of the same patients, indicating lineage-specific adaptation to venetoclax therapy.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



Comment in
-
A BAX door to venetoclax resistance.Blood. 2022 Feb 24;139(8):1124-1126. doi: 10.1182/blood.2021013788. Blood. 2022. PMID: 35201333 No abstract available.
References
-
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. - PubMed
-
- Kollek M, Müller A, Egle A, Erlacher M. Bcl-2 proteins in development, health, and disease of the hematopoietic system. FEBS J. 2016;283(15):2779–2810. - PubMed
-
- Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

