The Global Landscape of Pediatric Bacterial Meningitis Data Reported to the World Health Organization-Coordinated Invasive Bacterial Vaccine-Preventable Disease Surveillance Network, 2014-2019
- PMID: 34469555
- PMCID: PMC8409679
- DOI: 10.1093/infdis/jiab217
The Global Landscape of Pediatric Bacterial Meningitis Data Reported to the World Health Organization-Coordinated Invasive Bacterial Vaccine-Preventable Disease Surveillance Network, 2014-2019
Abstract
Background: The World Health Organization (WHO) coordinates the Global Invasive Bacterial Vaccine-Preventable Diseases (IB-VPD) Surveillance Network to support vaccine introduction decisions and use. The network was established to strengthen surveillance and laboratory confirmation of meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis.
Methods: Sentinel hospitals report cases of children <5 years of age hospitalized for suspected meningitis. Laboratories report confirmatory testing results and strain characterization tested by polymerase chain reaction. In 2019, the network included 123 laboratories that follow validated, standardized testing and reporting strategies.
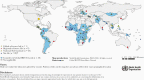
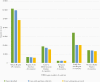
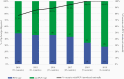
Results: From 2014 through 2019, >137 000 suspected meningitis cases were reported by 58 participating countries, with 44.6% (n = 61 386) reported from countries in the WHO African Region. More than half (56.6%, n = 77 873) were among children <1 year of age, and 4.0% (n = 4010) died among those with reported disease outcome. Among suspected meningitis cases, 8.6% (n = 11 798) were classified as probable bacterial meningitis. One of 3 bacterial pathogens was identified in 30.3% (n = 3576) of these cases, namely S. pneumoniae (n = 2177 [60.9%]), H. influenzae (n = 633 [17.7%]), and N. meningitidis (n = 766 [21.4%]). Among confirmed bacterial meningitis cases with outcome reported, 11.0% died; case fatality ratio varied by pathogen (S. pneumoniae, 12.2%; H. influenzae, 6.1%; N. meningitidis, 11.0%). Among the 277 children who died with confirmed bacterial meningitis, 189 (68.2%) had confirmed S. pneumoniae. The proportion of pneumococcal cases with pneumococcal conjugate vaccine (PCV) serotypes decreased as the number of countries implementing PCV increased, from 77.8% (n = 273) to 47.5% (n = 248). Of 397 H. influenzae specimens serotyped, 49.1% (n = 195) were type b. Predominant N. meningitidis serogroups varied by region.
Conclusions: This multitier, global surveillance network has supported countries in detecting and serotyping the 3 principal invasive bacterial pathogens that cause pediatric meningitis. Streptococcus pneumoniae was the most common bacterial pathogen detected globally despite the growing number of countries that have nationally introduced PCV. The large proportions of deaths due to S. pneumoniae reflect the high proportion of meningitis cases caused by this pathogen. This global network demonstrated a strong correlation between PCV introduction status and reduction in the proportion of pneumococcal meningitis infections caused by vaccine serotypes. Maintaining case-based, active surveillance with laboratory confirmation for prioritized vaccine-preventable diseases remains a critical component of the global agenda in public health.The World Health Organization (WHO)-coordinated Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance Network reported data from 2014 to 2019, contributing to the estimates of the disease burden and serotypes of pediatric meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis.
Keywords: invasive bacterial disease; meningitis; meningococcal; pneumococcal; pneumococcal conjugate vaccine; surveillance; vaccine preventable disease.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Trotter CL, Lingani C, Fernandez K, et al. . Impact of MenAfriVac in nine countries of the African meningitis belt, 2010-15: an analysis of surveillance data. Lancet Infect Dis 2017; 17:867–72. - PubMed
-
- World Health Organization. WHO global invasive bacterial vaccine-preventable disease and rotavirus and pediatric diarrhea surveillance networks bulletin, January 2020. 2020. http://www.who.int/immunization/monitoring_surveillance/resources/NUVI/en/. Accessed 15 May 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

