High Prevalence of Vaccine-Type Infections Among Children with Pneumococcal Pneumonia and Effusion After 13-Valent Pneumococcal Conjugate Vaccine Introduction in the Dominican Republic
- PMID: 34469563
- PMCID: PMC8414918
- DOI: 10.1093/infdis/jiab134
High Prevalence of Vaccine-Type Infections Among Children with Pneumococcal Pneumonia and Effusion After 13-Valent Pneumococcal Conjugate Vaccine Introduction in the Dominican Republic
Abstract
Background: In 2013, the Dominican Republic introduced 13-valent pneumococcal conjugate vaccine (PCV13) using a 3-dose schedule (at 2, 4 and 12 months of age). We evaluated the impact of PCV13 on serotypes causing pneumococcal pneumonia with pleural effusion.
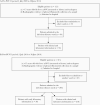
Methods: Surveillance data after PCV13 introduction (July 2014 to June 2016) were compared with data before PCV13 introduction (July 2009 to June 2011). Cases were defined as radiologic evidence of pneumonia with pleural effusion in a child aged <15 years. Pneumococcus was detected in pleural fluid by either culture or polymerase chain reaction, and serotyping was performed. The Ministry of Health's PCV13 uptake data for 2014-2016 were obtained.
Results: The prevalence of pneumococcus among cases was similar before and after PCV13 introduction (56.4% and 52.8%, respectively). The proportion of pneumococcal cases caused by vaccine serotypes was 86% for children <2 years old both before and PCV13 introduction. Compared with before PCV13, serotype 14 accounted for a smaller (28% vs 13%, respectively; P = .02) and serotype 1 for a larger (23% vs 37%; P = .09) proportion of pneumococcal cases after PCV13 introduction. National uptake for the first, second, and third PCV13 doses was 94%, 81%, and 28%, respectively, in 2014 and 75%, 61%, and 26% in 2015.
Discussion: While the decrease in pneumococcal pneumonia with pleural effusion caused by serotype 14 may reflect an early effect of PCV13 implementation, other vaccine serotypes, including serotype 1, are not well controlled. Better PCV13 coverage for all 3 doses is needed.
Keywords: 13-valent pneumococcal vaccine; Dominican Republic; pleural effusion; pneumococcal pneumonia.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures


References
-
- Liu L, Oza S, Hogan D, et al. . Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. - PubMed
-
- Valenzuela MT, O’Loughlin R, De La Hoz F, et al. . The burden of pneumococcal disease among Latin American and Caribbean children: review of the evidence. Rev Panam Salud Publica 2009; 25:270–9. - PubMed
-
- Sinha A, Constenla D, Valencia JE, et al. . Cost-effectiveness of pneumococcal conjugate vaccination in Latin America and the Caribbean: a regional analysis. Rev Panam Salud Publica 2008; 24:304–13. - PubMed
-
- World Health Organization. Pneumococcal vaccines: WHO position paper—2012. Wkly Epidemiol Rec 2012; 87:129–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

