AgRP neurons: Regulators of feeding, energy expenditure, and behavior
- PMID: 34469623
- PMCID: PMC9040143
- DOI: 10.1111/febs.16176
AgRP neurons: Regulators of feeding, energy expenditure, and behavior
Abstract
Neurons in the hypothalamic arcuate nucleus (ARC) that express agouti-related peptide (AgRP) govern a critical aspect of survival: the drive to eat. Equally important to survival is the timing at which food is consumed-seeking or eating food to alleviate hunger in the face of a more pressing threat, like the risk of predation, is clearly maladaptive. To ensure optimal prioritization of behaviors within a given environment, therefore, AgRP neurons must integrate signals of internal need states with contextual environmental cues. In this state-of-the-art review, we highlight recent advances that extend our understanding of AgRP neurons, including the neural circuits they engage to regulate feeding, energy expenditure, and behavior. We also discuss key findings that illustrate how both classical feedback and anticipatory feedforward signals regulate this neuronal population and how the integration of these signals may be disrupted in states of energy excess. Finally, we examine both technical and conceptual challenges facing the field moving forward.
Keywords: agouti-related peptide (AgRP); arcuate nucleus; behavior; energy expenditure; energy homeostasis; food intake; hunger; neurocircuits.
© 2021 Federation of European Biochemical Societies.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures

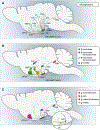
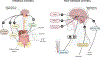

References
-
- Sternson SM & Eiselt A-K (2015) Three pillars for the neural control of appetite. Annu Rev Physiol 79, 401–423. - PubMed
-
- Sutton AK & Krashes MJ (2020) Integrating hunger with rival motivations. Trends Endocrinol Metab 31, 495–507. - PubMed
-
- Sternson SM (2020) ScienceDirect Exploring internal state-coding across the rodent brain. Curr Opin Neurobiol 65,20–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

