A Randomized Clinical Trial of Antimicrobial Duration for Cystic Fibrosis Pulmonary Exacerbation Treatment
- PMID: 34469706
- PMCID: PMC8786075
- DOI: 10.1164/rccm.202102-0461OC
A Randomized Clinical Trial of Antimicrobial Duration for Cystic Fibrosis Pulmonary Exacerbation Treatment
Abstract
Rationale: People with cystic fibrosis (CF) experience acute worsening of respiratory symptoms and lung function known as pulmonary exacerbations. Treatment with intravenous antimicrobials is common; however, there is scant evidence to support a standard treatment duration. Objectives: To test differing durations of intravenous antimicrobials for CF exacerbations. Methods: STOP2 (Standardized Treatment of Pulmonary Exacerbations 2) was a multicenter, randomized, controlled clinical trial in exacerbations among adults with CF. After 7-10 days of treatment, participants exhibiting predefined lung function and symptom improvements were randomized to 10 or 14 days' total antimicrobial duration; all others were randomized to 14 or 21 days' duration. Measurements and Main Results: The primary outcome was percent predicted FEV1 (ppFEV1) change from treatment initiation to 2 weeks after cessation. Among early responders, noninferiority of 10 days to 14 days was tested; superiority of 21 days compared with 14 days was compared for the others. Symptoms, weight, and adverse events were secondary. Among 982 randomized people, 277 met improvement criteria and were randomized to 10 or 14 days of treatment; the remaining 705 received 21 or 14 days of treatment. Mean ppFEV1 change was 12.8 and 13.4 for 10 and 14 days, respectively, a ‒0.65 difference (95% CI [‒3.3 to 2.0]), excluding the predefined noninferiority margin. The 21- and 14-day arms experienced 3.3 and 3.4 mean ppFEV1 changes, a difference of ‒0.10 (‒1.3 to 1.1). Secondary endpoints and sensitivity analyses were supportive. Conclusions: Among adults with CF with early treatment improvement during exacerbation, ppFEV1 after 10 days of intravenous antimicrobials is not inferior to 14 days. For those with less improvement after one week, 21 days is not superior to 14 days. Clinical trial registered with www.clinicaltrials.gov (NCT02781610).
Keywords: respiratory infection; clinical trial; intravenous antibiotic therapy.
Figures
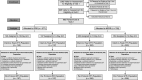


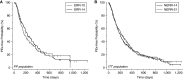
Comment in
-
Two Steps Forward: Improving the Management of Cystic Fibrosis Pulmonary Exacerbations.Am J Respir Crit Care Med. 2021 Dec 1;204(11):1245-1247. doi: 10.1164/rccm.202108-1939ED. Am J Respir Crit Care Med. 2021. PMID: 34543576 Free PMC article. No abstract available.
References
-
- Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc . 2016;13:1173–1179. - PubMed
-
- Jain M, Goss CH. Update in cystic fibrosis 2013. Am J Respir Crit Care Med . 2014;189:1181–1186. - PubMed
-
- Elborn JS. Cystic fibrosis. Lancet . 2016;388:2519–2531. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

