Evaluation of autophagy mediators in myeloid-derived suppressor cells during human tuberculosis
- PMID: 34469846
- PMCID: PMC9278564
- DOI: 10.1016/j.cellimm.2021.104426
Evaluation of autophagy mediators in myeloid-derived suppressor cells during human tuberculosis
Abstract
Myeloid-derived suppressor cells (MDSC) are induced during active TB disease to restore immune homeostasis but instead exacerbate disease outcome due to chronic inflammation. Autophagy, in conventional phagocytes, ensures successful clearance of M.tb. However, autophagy has been demonstrated to induce prolonged MDSC survival. Here we investigate the relationship between autophagy mediators and MDSC in the context of active TB disease and during anti-TB therapy. We demonstrate a significant increase in MDSC frequencies in untreated active TB cases with these MDSC expressing TLR4 and significantly more mTOR and IL-6 than healthy controls, with mTOR levels decreasing during anti-TB therapy. Finally, we show that HMGB1 serum concentrations decrease in parallel with mTOR. These findings suggest a complex interplay between MDSC and autophagic mediators, potentially dependent on cellular localisation and M.tb infection state.
Keywords: Autophagy; High mobility group box protein 1; Mycobacterium tuberculosis; Myeloid-derived suppressor cells; Tuberculosis.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no financial conflicts of interest.
Figures


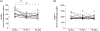

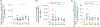
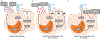
References
-
- Talmadge JE, Cole K, Britton H, Dafferner A, and Warkentin P, “Human myeloid derived suppressor cell (MDSC) subset phenotypes,” J. Immunol, vol. 198, no. 1 Supplement, p. 211.2–211.2, May 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

