Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4)
- PMID: 34470477
- PMCID: PMC8545254
- DOI: 10.1161/ATVBAHA.121.316600
Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4)
Abstract
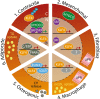
Multiple layers of vascular smooth muscle cells (vSMCs) are present in blood vessels forming the media of the vessel wall. vSMCs provide a vessel wall structure, enabling it to contract and relax, thus modulating blood flow. They also play a crucial role in the development of vascular diseases, such as atherosclerosis and aortic aneurysm formation. vSMCs display a remarkable high degree of plasticity. At present, the number of different vSMC phenotypes has only partially been characterized. By mapping vSMC phenotypes in detail and identifying triggers for phenotype switching, the relevance of the different phenotypes in vascular disease may be identified. Up until recently, vSMCs were classified as either contractile or dedifferentiated (ie, synthetic). However, single-cell RNA sequencing studies revealed such dedifferentiated arterial vSMCs to be highly diverse. Currently, no consensus exist about the number of vSMC phenotypes. Therefore, we reviewed the data from relevant single-cell RNA sequencing studies, and classified a total of 6 vSMC phenotypes. The central dedifferentiated vSMC type that we classified is the mesenchymal-like phenotype. Mesenchymal-like vSMCs subsequently seem to differentiate into fibroblast-like, macrophage-like, osteogenic-like, and adipocyte-like vSMCs, which contribute differentially to vascular disease. This phenotype switching between vSMCs requires the transcription factor KLF4 (Kruppel-like factor 4). Here, we performed an integrated analysis of the data about the recently identified vSMC phenotypes, their associated gene expression profiles, and previous vSMC knowledge to better understand the role of vSMC phenotype transitions in vascular pathology.
Keywords: atherosclerosis; cardiovascular disease; myocytes; phenotype; smooth muscle.
Figures

References
-
- Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320 - PubMed
-
- Kellogg DL., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol (1985). 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005 - PubMed
-
- Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M, Jørgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567. doi: 10.1038/s41467-018-06891-x - PMC - PubMed
-
- Pedroza AJ, Tashima Y, Shad R, Cheng P, Wirka R, Churovich S, Nakamura K, Yokoyama N, Cui JZ, Iosef C, et al. . Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol. 2020;40:2195–2211. doi: 10.1161/ATVBAHA.120.314670 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

