Novel Mouse Models of Bladder Cancer Identify a Prognostic Signature Associated with Risk of Disease Progression
- PMID: 34470779
- PMCID: PMC8609963
- DOI: 10.1158/0008-5472.CAN-21-1254
Novel Mouse Models of Bladder Cancer Identify a Prognostic Signature Associated with Risk of Disease Progression
Abstract
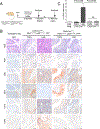
To study the progression of bladder cancer from non-muscle-invasive to muscle-invasive disease, we have developed a novel toolkit that uses complementary approaches to achieve gene recombination in specific cell populations in the bladder urothelium in vivo, thereby allowing us to generate a new series of genetically engineered mouse models (GEMM) of bladder cancer. One method is based on the delivery of adenoviruses that express Cre recombinase in selected cell types in the urothelium, and a second uses transgenic drivers in which activation of inducible Cre alleles can be limited to the bladder urothelium by intravesicular delivery of tamoxifen. Using both approaches, targeted deletion of the Pten and p53 tumor suppressor genes specifically in basal urothelial cells gave rise to muscle-invasive bladder tumors. Furthermore, preinvasive lesions arising in basal cells displayed upregulation of molecular pathways related to bladder tumorigenesis, including proinflammatory pathways. Cross-species analyses comparing a mouse gene signature of early bladder cancer with a human signature of bladder cancer progression identified a conserved 28-gene signature of early bladder cancer that is associated with poor prognosis for human bladder cancer and that outperforms comparable gene signatures. These findings demonstrate the relevance of these GEMMs for studying the biology of human bladder cancer and introduce a prognostic gene signature that may help to stratify patients at risk for progression to potentially lethal muscle-invasive disease. SIGNIFICANCE: Analyses of bladder cancer progression in a new series of genetically engineered mouse models has identified a gene signature of poor prognosis in human bladder cancer.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures

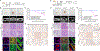


References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33 - PubMed
-
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41 - PubMed
-
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet 2016 - PubMed
-
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin 2020;70:404–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

