Improved modeling of human AD with an automated culturing platform for iPSC neurons, astrocytes and microglia
- PMID: 34471104
- PMCID: PMC8410795
- DOI: 10.1038/s41467-021-25344-6
Improved modeling of human AD with an automated culturing platform for iPSC neurons, astrocytes and microglia
Abstract
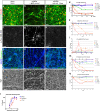
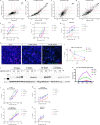
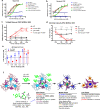
Advancement in human induced pluripotent stem cell (iPSC) neuron and microglial differentiation protocols allow for disease modeling using physiologically relevant cells. However, iPSC differentiation and culturing protocols have posed challenges to maintaining consistency. Here, we generated an automated, consistent, and long-term culturing platform of human iPSC neurons, astrocytes, and microglia. Using this platform we generated a iPSC AD model using human derived cells, which showed signs of Aβ plaques, dystrophic neurites around plaques, synapse loss, dendrite retraction, axon fragmentation, phospho-Tau induction, and neuronal cell death in one model. We showed that the human iPSC microglia internalized and compacted Aβ to generate and surround the plaques, thereby conferring some neuroprotection. We investigated the mechanism of action of anti-Aβ antibodies protection and found that they protected neurons from these pathologies and were most effective before pTau induction. Taken together, these results suggest that this model can facilitate target discovery and drug development efforts.
© 2021. The Author(s).
Conflict of interest statement
All authors were employees of Genentech during the course of these studies.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

