Non-respiratory particles emitted by guinea pigs in airborne disease transmission experiments
- PMID: 34471147
- PMCID: PMC8410799
- DOI: 10.1038/s41598-021-96678-w
Non-respiratory particles emitted by guinea pigs in airborne disease transmission experiments
Abstract
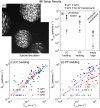
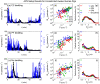
Animal models are often used to assess the airborne transmissibility of various pathogens, which are typically assumed to be carried by expiratory droplets emitted directly from the respiratory tract of the infected animal. We recently established that influenza virus is also transmissible via "aerosolized fomites," micron-scale dust particulates released from virus-contaminated surfaces (Asadi et al. in Nat Commun 11(1):4062, 2020). Here we expand on this observation, by counting and characterizing the particles emitted from guinea pig cages using an Aerodynamic Particle Sizer (APS) and an Interferometric Mie Imaging (IMI) system. Of over 9000 airborne particles emitted from guinea pig cages and directly imaged with IMI, none had an interference pattern indicative of a liquid droplet. Separate measurements of the particle count using the APS indicate that particle concentrations spike upwards immediately following animal motion, then decay exponentially with a time constant commensurate with the air exchange rate in the cage. Taken together, the results presented here raise the possibility that a non-negligible fraction of airborne influenza transmission events between guinea pigs occurs via aerosolized fomites rather than respiratory droplets, though the relative frequencies of these two routes have yet to be definitively determined.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

