Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder
- PMID: 34471251
- PMCID: PMC8872999
- DOI: 10.1038/s41380-021-01268-x
Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder
Abstract
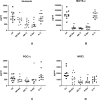
To characterize neuronal mitochondrial abnormalities in major depressive disorder (MDD), functional mitochondrial proteins (MPs) extracted from enriched plasma neuron-derived extracellular vesicles (NDEVs) of MDD participants (n = 20) were quantified before and after eight weeks of treatment with a selective serotonin reuptake inhibitor (SSRI). Pretreatment baseline NDEV levels of the transcriptional type 2 nuclear respiratory factor (NRF2) which controls mitochondrial biogenesis and many anti-oxidant gene responses, regulators of diverse neuronal mitochondrial functions cyclophilin D (CYPD) and mitofusin-2 (MFN2), leucine zipper EF-hand containing transmembrane 1 protein (LETM1) component of a calcium channel/calcium channel enhancer, mitochondrial tethering proteins syntaphilin (SNPH) and myosin VI (MY06), inner membrane electron transport complexes I (subunit 6) and III (subunit 10), the penultimate enzyme of nicotinamide adenine dinucleotide (NAD) generation nicotinamide mononucleotide adenylytransferase 2 (NMNAT2), and neuronal mitochondrial metabolic regulatory and protective factors humanin and mitochondrial open-reading frame of the 12S rRNA-c (MOTS-c) all were significantly lower than those of NDEVs from matched controls (n = 10), whereas those of pro-neurodegenerative NADase Sterile Alpha and TIR motif-containing protein 1 (SARM1) were higher. The baseline NDEV levels of transcription factor A mitochondrial (TFAM) and the transcriptional master-regulator of mitochondrial biogenesis PPAR γ coactivator-1α (PGC-1α) showed no differences between MDD participants and controls. Several of these potential biomarker proteins showed substantially different changes in untreated MDD than those we reported in untreated first-episode psychosis. NDEV levels of MPs of all functional classes, except complex I-6, NRF2 and PGC-1α were normalized in MDD participants who responded to SSRI therapy (n = 10) but not in those who failed to respond (n = 10) by psychiatric evaluation. If larger studies validate NDEV MP abnormalities, they may become useful biomarkers and identify new drug targets.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

