SARS-CoV-2-specific T cells in infection and vaccination
- PMID: 34471260
- PMCID: PMC8408362
- DOI: 10.1038/s41423-021-00743-3
SARS-CoV-2-specific T cells in infection and vaccination
Abstract
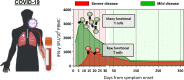
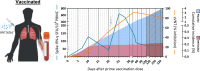
During viral infections, antibodies and T cells act together to prevent pathogen spread and remove virus-infected cells. Virus-specific adaptive immunity can, however, also trigger pathological processes characterized by localized or systemic inflammatory events. The protective and/or pathological role of virus-specific T cells in SARS-CoV-2 infection has been the focus of many studies in COVID-19 patients and in vaccinated individuals. Here, we review the works that have elucidated the function of SARS-CoV-2-specific T cells in patients and in vaccinated individuals. Understanding whether SARS-CoV-2-specific T cells are more linked to protection or pathogenesis is pivotal to define future therapeutic and prophylactic strategies to manage the current pandemic.
Keywords: COVID-19; SARS-CoV-2; T cells; Vaccines.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2020;181:1489–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

