Intrinsic Defects in LiMn2O4: First-Principles Calculations
- PMID: 34471730
- PMCID: PMC8388003
- DOI: 10.1021/acsomega.1c01162
Intrinsic Defects in LiMn2O4: First-Principles Calculations
Abstract
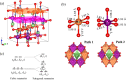
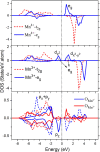
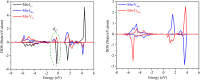
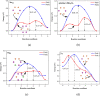
Spinel LiMn2O4 has attracted wide attention due to its advantages of a high-voltage plateau, good capacity, environmental friendliness, and low cost. Due to different experimental synthesis methods and conditions, there are many intrinsic point defects in LiMn2O4. By means of first-principles calculations based on a reasonable magnetic configuration, we studied the formation energies, local structures, and charge compensation mechanism of intrinsic point defects in LiMn2O4. The formation energies of defects under the assumed O-rich equilibrium conditions were examined. It was found that O, Li, and Mn vacancies, Mn and Li antisites, and Li interstitial could appear in the lattice at some equilibrium conditions, but Mn interstitial is hard to form. The charge was compensated mainly by adjusting the oxidation state of Mn around the defect, except for the defects at the 8a Wyckoff site. The binding energies between point defects were calculated to shed light on the clustering of point defects. Furthermore, the diffusion of Li ions around the defects was discussed. Cation antisites led to a decrease of the Li diffusion barrier but O vacancy caused an increase of the barrier. This study provides theoretical support for understanding point defects in spinel LiMn2O4.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Bäuerlein P.; Herr R.; Kloss M.; Kümpers J.; Maul M.; Meissner E. Advanced lithium ion cells with lithium manganese spinel. J. Power Sources 1999, 81–82, 585–588. 10.1016/S0378-7753(99)00226-8. - DOI
-
- Gummow R. J.; Kock A. D.; Thackeray M. M. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells. Solid State Ionics 1994, 69, 59–67. 10.1016/0167-2738(94)90450-2. - DOI
-
- Guyomard D.; Tarascon J. M. The carbon/Li1+xMn2O4 system. Solid State Ionics 1994, 69, 222–237. 10.1016/0167-2738(94)90412-X. - DOI
-
- Thackeray M.; David W.; Bruce P.; Goodenough J. B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. 10.1016/0025-5408(83)90138-1. - DOI
-
- Manev V.; Banov B.; Momchilov A.; Nassalevska A. LiMn2O4 for 4 V lithium-ion batteries. J. Power Sources 1995, 57, 99–103. 10.1016/0378-7753(95)02227-9. - DOI
LinkOut - more resources
Full Text Sources

