Reutilizing Methane Reforming Spent Catalysts as Efficient Overall Water-Splitting Electrocatalysts
- PMID: 34471736
- PMCID: PMC8388002
- DOI: 10.1021/acsomega.1c01558
Reutilizing Methane Reforming Spent Catalysts as Efficient Overall Water-Splitting Electrocatalysts
Abstract
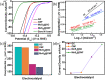
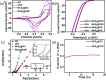
It is extremely prudent and highly challenging to design a greener bifunctional electrocatalyst that shows effective electrocatalytic activity and high stability toward electrochemical water splitting. As several hundred tons of catalysts are annually deactivated by deposition of carbon, herein, we came up with a strategy to reutilize spent methane reforming catalysts that were deactivated by the formation of graphitic carbon (GC) and carbon nanofibers (CNF). An electrocatalyst was successfully synthesized by in situ deposition of noble metal-free MoS2 over spent catalysts via a hydrothermal method that showed exceptional performance regarding the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). At 25 mA cm-2, phenomenal OER overpotentials (η25) of 128 and 154 mV and modest HER overpotentials of 186 and 207 mV were achieved for MoS2@CNF and MoS2@GC, respectively. Moreover, OER Tafel slopes of 41 and 71 mV dec-1 and HER Tafel slopes of 99 and 107 mV dec-1 were obtained for MoS2@CNF and MoS2@GC, respectively. Furthermore, the synthesized catalysts exhibited good long-term durability for about 18 h at 100 μA cm-2 with unnoticeable changes in the linear sweep voltammetry (LSV) curve of the HER after 1000 cycles. The carbon on the spent catalyst increased the conductivity, while MoS2 enhanced the electrocatalytic activity; hence, the synergistic effect of both materials resulted in enhanced electrocatalysts for overall water splitting. This work of synthesizing enhanced nanostructured electrocatalysts with minimal usage of inexpensive MoS2 gives a rationale for engineering potent greener electrocatalysts.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Shukla P. R.; Skea J.; Calvo Buendia E.; Masson-Delmotte V.; Pörtner H. O.; Roberts D. C.; Zhai P.; Slade R.; Connors S.; Van Diemen R.. et al.Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change (IPCC), 2019.
-
- Staffell I.; Scamman D.; Abad A. V.; Balcombe P.; Dodds P. E.; Ekins P.; Shah N.; Ward K. R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. 10.1039/C8EE01157E. - DOI
-
- IEA. Hydrogen; International Energy Agency IEA, Paris, 2020; p 203.
-
- Yu S.-B.; Lee S.-H.; Mehran M. T.; Hong J.-E.; Lee J.-W.; Lee S.-B.; Park S.-J.; Song R.-H.; Shim J.-H.; Shul Y.-G.; Lim T.-H. Syngas Production in High Performing Tubular Solid Oxide Cells by Using High-Temperature H2O/CO2 Co-Electrolysis. Chem. Eng. J. 2018, 335, 41–51. 10.1016/j.cej.2017.10.110. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

