Electroconductive Nanobiomaterials for Tissue Engineering and Regenerative Medicine
- PMID: 34471843
- PMCID: PMC8370325
- DOI: 10.1089/bioe.2020.0021
Electroconductive Nanobiomaterials for Tissue Engineering and Regenerative Medicine
Abstract
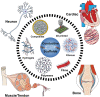
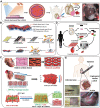
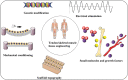
Regenerative medicine aims to engineer tissue constructs that can recapitulate the functional and structural properties of native organs. Most novel regenerative therapies are based on the recreation of a three-dimensional environment that can provide essential guidance for cell organization, survival, and function, which leads to adequate tissue growth. The primary motivation in the use of conductive nanomaterials in tissue engineering has been to develop biomimetic scaffolds to recapitulate the electrical properties of the natural extracellular matrix, something often overlooked in numerous tissue engineering materials to date. In this review article, we focus on the use of electroconductive nanobiomaterials for different biomedical applications, particularly, very recent advancements for cardiovascular, neural, bone, and muscle tissue regeneration. Moreover, this review highlights how electroconductive nanobiomaterials can facilitate cell to cell crosstalk (i.e., for cell growth, migration, proliferation, and differentiation) in different tissues. Thoughts on what the field needs for future growth are also provided.
Keywords: biomaterials; bone; cardiac; electroconductive; extracellular matrix; nanomaterials; nanomedicine; nerve; regenerative medicine; tendon; tissue engineering.
Copyright 2020, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Liu Y, Lim J, Teoh S-H. Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv 2013;31:688–705 - PubMed
-
- Hosoyama K, Ahumada M, Goel K, et al. . Electroconductive materials as biomimetic platforms for tissue regeneration. Biotechnol Adv 2019;37:444–458 - PubMed
-
- Bassett CAL, Becker RO. Generation of electric potentials by bone in response to mechanical stress. Science 1962;137:1063–1064 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
