Discovery of Novel Acetamide-Based Heme Oxygenase-1 Inhibitors with Potent In Vitro Antiproliferative Activity
- PMID: 34472337
- PMCID: PMC8474116
- DOI: 10.1021/acs.jmedchem.1c00633
Discovery of Novel Acetamide-Based Heme Oxygenase-1 Inhibitors with Potent In Vitro Antiproliferative Activity
Abstract
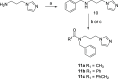
Heme oxygenase-1 (HO-1) promotes heme catabolism exercising cytoprotective roles in normal and cancer cells. Herein, we report the design, synthesis, molecular modeling, and biological evaluation of novel HO-1 inhibitors. Specifically, an amide linker in the central spacer and an imidazole were fixed, and the hydrophobic moiety required by the pharmacophore was largely modified. In many tumors, overexpression of HO-1 correlates with poor prognosis and chemoresistance, suggesting the inhibition of HO-1 as a possible antitumor strategy. Accordingly, compounds 7i and 7l-p emerged for their potency against HO-1 and were investigated for their anticancer activity against prostate (DU145), lung (A549), and glioblastoma (U87MG, A172) cancer cells. The selected compounds showed the best activity toward U87MG cells. Compound 7l was further investigated for its in-cell enzymatic HO-1 activity, expression levels, and effects on cell invasion and vascular endothelial growth factor (VEGF) extracellular release. The obtained data suggest that 7l can reduce cell invasivity acting through modulation of HO-1 expression.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















References
-
- Sorrenti V.; Raffaele M.; Vanella L.; Acquaviva R.; Salerno L.; Pittala V.; Intagliata S.; Di Giacomo C. Protective effects of caffeic acid phenethyl ester (CAPE) and novel cape analogue as inducers of heme oxygenase-1 in streptozotocin-induced type 1 diabetic rats. Int. J. Mol. Sci. 2019, 20, 244110.3390/ijms20102441. - DOI - PMC - PubMed
-
- Pittalà V.; Vanella L.; Platania C. B. M.; Salerno L.; Raffaele M.; Amata E.; Marrazzo A.; Floresta G.; Romeo G.; Greish K.; Intagliata S.; Bucolo C.; Sorrenti V. Synthesis, in vitro and in silico studies of HO-1 inducers and lung antifibrotic agents. Future Med. Chem. 2019, 11, 1523–1536. 10.4155/fmc-2018-0448. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

