Vitamin K - sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity
- PMID: 34472618
- PMCID: PMC8907489
- DOI: 10.1093/nutrit/nuab061
Vitamin K - sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity
Abstract
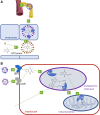
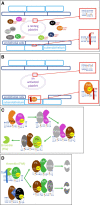
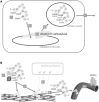
Vitamin K is traditionally connected with blood coagulation, since it is needed for the posttranslational modification of 7 proteins involved in this cascade. However, it is also involved in the maturation of another 11 or 12 proteins that play different roles, encompassing in particular the modulation of the calcification of connective tissues. Since this process is physiologically needed in bones, but is pathological in arteries, a great deal of research has been devoted to finding a possible link between vitamin K and the prevention of osteoporosis and cardiovascular diseases. Unfortunately, the current knowledge does not allow us to make a decisive conclusion about such a link. One possible explanation for this is the diversity of the biological activity of vitamin K, which is not a single compound but a general term covering natural plant and animal forms of vitamin K (K1 and K2) as well as their synthetic congeners (K3 and K4). Vitamin K1 (phylloquinone) is found in several vegetables. Menaquinones (MK4-MK13, a series of compounds known as vitamin K2) are mostly of a bacterial origin and are introduced into the human diet mainly through fermented cheeses. Current knowledge about the kinetics of different forms of vitamin K, their detection, and their toxicity are discussed in this review.
Keywords: bioanalysis; coagulation; diet; menaquinone; phylloquinone.
© The Author(s) 2021. Published by Oxford University Press on behalf of the International Life Sciences Institute.
Figures


References
-
- Raju TNK. The Nobel Chronicles. Lancet .1999;353:761. - PubMed
-
- Tarento TDC, McClure DD, Dehghani F, et al.Pilot-scale production of phylloquinone (vitamin K1) using a bubble column photo-bioreactor. Biochem Eng J. 2019;150:107243.
-
- Tarento TDC, McClure DD, Talbot AM, et al.A potential biotechnological process for the sustainable production of vitamin K1. Crit Rev Biotechnol .2019;39:1–19. - PubMed
-
- Manzotti P, Nisi P, Zocchi G.. Vitamin K in plants. Funct Plant Sci Biotechnol. 2008;2:29–35.

